Establishment and transfer of classical eyeblink conditioning using electrical microstimulation of the hippocampus as the conditioned stimulus
- PMID: 28575003
- PMCID: PMC5456086
- DOI: 10.1371/journal.pone.0178502
Establishment and transfer of classical eyeblink conditioning using electrical microstimulation of the hippocampus as the conditioned stimulus
Abstract
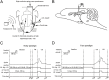
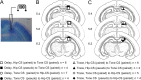
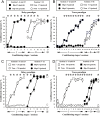
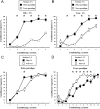
The present experiment was designed to determine whether classical eyeblink conditioning (EBC) can be established by using electrical microstimulation of the hippocampus as a conditioned stimulus (CS) paired with an air-puff unconditioned stimulus (US). We intended to examine whether EBC transfer could occur when a CS was shifted between microstimulation of the hippocampus as a CS (Hip-CS) and tone as a CS (tone-CS) and to compare the difference in transfer effectiveness between delay EBC (dEBC) and trace EBC (tEBC). Eight groups of guinea pigs, including 4 experimental groups and 4 control groups, were included in the study. First, the experimental groups received either a Hip-CS or a tone-CS paired with a US; then, these groups were exposed to a shifted CS (tone-CS or Hip-CS) paired with the US. The control groups received the corresponding Hip-CS or tone-CS, which was, however, pseudo-paired with the US. The control groups were then shifted to the tone-CS (or Hip-CS) paired with the US. The results show that EBC can be successfully established when using microstimulation of the hippocampus as a CS paired with an air-puff US, and that the acquisition rates of EBC are higher in the experimental groups than in the control groups after switching from the Hip-CS to the tone-CS or vice versa, indicating the occurrence of learning transfer between EBC established with the Hip-CS and tone-CS. The present study also demonstrated that the EBC re-acquisition rates were remarkably higher in dEBC than in tEBC with both types of transfer, which suggests that the saving effect was more evident in dEBC than tEBC. These results significantly expand our knowledge of EBC transfer as well as the functional neural circuit underlying EBC transfer.
Conflict of interest statement
Figures
Similar articles
-
Transfer of classical eyeblink conditioning with electrical stimulation of mPFC or tone as conditioned stimulus in guinea pigs.Behav Brain Res. 2014 Nov 1;274:19-29. doi: 10.1016/j.bbr.2014.07.051. Epub 2014 Aug 10. Behav Brain Res. 2014. PMID: 25106738
-
Modulation of eyeblink conditioning through sensory processing of conditioned stimulus by cortical and subcortical regions.Behav Brain Res. 2019 Feb 1;359:149-155. doi: 10.1016/j.bbr.2018.10.035. Epub 2018 Oct 29. Behav Brain Res. 2019. PMID: 30385367 Review.
-
CS-US preexposure effects on trace eyeblink conditioning in young rats: potential implications for functional brain development.Behav Neurosci. 2006 Apr;120(2):257-66. doi: 10.1037/0735-7044.120.2.257. Behav Neurosci. 2006. PMID: 16719690
-
Sustained Activity of Hippocampal Parvalbumin-Expressing Interneurons Supports Trace Eyeblink Conditioning in Mice.J Neurosci. 2022 Nov 2;42(44):8343-8360. doi: 10.1523/JNEUROSCI.0834-22.2022. Epub 2022 Sep 27. J Neurosci. 2022. PMID: 36167784 Free PMC article.
-
Hippocampal encoding of non-spatial trace conditioning.Hippocampus. 1999;9(4):385-96. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. Hippocampus. 1999. PMID: 10495020 Review.
References
-
- DOTY RW. Conditioned reflexes formed and evoked by brain stimulation. Electrical stimulation of the brain. 1961:397–412.
-
- Doty RW. Conditioned Reflexes Elicited by Electrical Stimulation of the Brain in Macaques. Journal of neurophysiology. 1965;28:623–40. - PubMed
-
- Doty RW. Electrical stimulation of the brain in behavioral context. Annual review of psychology. 1969;20:289–320. doi: 10.1146/annurev.ps.20.020169.001445 - DOI - PubMed
-
- Doty RW, Rutledge LT. Generalization between cortically and peripherally applied stimuli eliciting conditioned reflexes. Journal of neurophysiology. 1959;22(4):428–35. - PubMed
-
- Grigoryan GA. Generalization of the conditioned reflexes established by direct electrical stimulation of the hippocampus. Acta neurobiologiae experimentalis. 1981;41(5):423–37. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

