FOXC1 modulates MYOC secretion through regulation of the exocytic proteins RAB3GAP1, RAB3GAP2 and SNAP25
- PMID: 28575017
- PMCID: PMC5456087
- DOI: 10.1371/journal.pone.0178518
FOXC1 modulates MYOC secretion through regulation of the exocytic proteins RAB3GAP1, RAB3GAP2 and SNAP25
Abstract
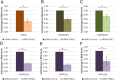
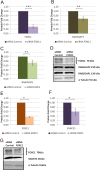
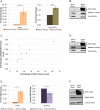
The neurodegenerative disease glaucoma is one of the leading causes of blindness in the world. Glaucoma is characterized by progressive visual field loss caused by retinal ganglion cell (RGC) death. Both surgical glaucoma treatments and medications are available, however, they only halt glaucoma progression and are unable to reverse damage. Furthermore, many patients do not respond well to treatments. It is therefore important to better understand the mechanisms involved in glaucoma pathogenesis. Patients with Axenfeld-Rieger syndrome (ARS) offer important insight into glaucoma progression. ARS patients are at 50% risk of developing early onset glaucoma and respond poorly to treatments, even when surgical treatments are combined with medications. Mutations in the transcription factor FOXC1 cause ARS. Alterations in FOXC1 levels cause ocular malformations and disrupt stress response in ocular tissues, thereby contributing to glaucoma progression. In this study, using biochemical and molecular techniques, we show that FOXC1 regulates the expression of RAB3GAP1, RAB3GAP2 and SNAP25, three genes with central roles in both exocytosis and endocytosis, responsible for extracellular trafficking. FOXC1 positively regulates RAB3GAP1 and RAB3GAP2, while either increase or decrease in FOXC1 levels beyond its normal range results in decreased SNAP25. In addition, we found that FOXC1 regulation of RAB3GAP1, RAB3GAP2 and SNAP25 affects secretion of Myocilin (MYOC), a protein associated with juvenile onset glaucoma and steroid-induced glaucoma. The present work reveals that FOXC1 is an important regulator of exocytosis and establishes a new link between FOXC1 and MYOC-associated glaucoma.
Conflict of interest statement
Figures








References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. Elsevier Inc; 2014;121: 2081–2090. doi: 10.1016/j.ophtha.2014.05.013 - DOI - PubMed
-
- Broman AT, Quigley HA, West SK, Katz J, Munoz B, Bandeen-Roche K, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Investig Ophthalmol {&} Vis Sci. 2008;49: 66–76. doi: 10.1167/iovs.07-0866 - DOI - PMC - PubMed
-
- Quigley HA. Glaucoma. Lancet. 2011;377: 1367–1377. doi: 10.1016/S0140-6736(10)61423-7 - DOI - PubMed
-
- Cairns JE. Trabeculectomy. Am J Ophthalmol. Elsevier; 1968;66: 673–679. - PubMed
-
- Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin Pharmacother. 2012;13: 723–745. doi: 10.1517/14656566.2012.662219 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

