High Rate of Treatment Completion in Program Settings With 12-Dose Weekly Isoniazid and Rifapentine for Latent Mycobacterium tuberculosis Infection
- PMID: 28575208
- PMCID: PMC5709238
- DOI: 10.1093/cid/cix505
High Rate of Treatment Completion in Program Settings With 12-Dose Weekly Isoniazid and Rifapentine for Latent Mycobacterium tuberculosis Infection
Abstract
Background: Randomized controlled trials have demonstrated that the newest latent tuberculosis (LTBI) regimen, 12 weekly doses of directly observed isoniazid and rifapentine (3HP), is as efficacious as 9 months of isoniazid, with a greater completion rate (82% vs 69%); however, 3HP has not been assessed in routine healthcare settings.
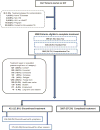
Methods: Observational cohort of LTBI patients receiving 3HP through 16 US programs was used to assess treatment completion, adverse drug reactions, and factors associated with treatment discontinuation.
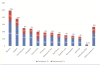
Results: Of 3288 patients eligible to complete 3HP, 2867 (87.2%) completed treatment. Children aged 2-17 years had the highest completion rate (94.5% [155/164]). Patients reporting homelessness had a completion rate of 81.2% (147/181). In univariable analyses, discontinuation was lowest among children (relative risk [RR], 0.44 [95% confidence interval {CI}, .23-.85]; P = .014), and highest in persons aged ≥65 years (RR, 1.72 [95% CI, 1.25-2.35]; P < .001). In multivariable analyses, discontinuation was lowest among contacts of patients with tuberculosis (TB) disease (adjusted RR [ARR], 0.68 [95% CI, .52-.89]; P = .005) and students (ARR, 0.45 [95% CI, .21-.98]; P = .044), and highest with incarceration (ARR, 1.43 [95% CI, 1.08-1.89]; P = .013) and homelessness (ARR, 1.72 [95% CI, 1.25-2.39]; P = .001). Adverse drug reactions were reported by 1174 (35.7%) patients, of whom 891 (76.0%) completed treatment.
Conclusions: Completion of 3HP in routine healthcare settings was greater overall than rates reported from clinical trials, and greater than historically observed using other regimens among reportedly nonadherent populations. Widespread use of 3HP for LTBI treatment could accelerate elimination of TB disease in the United States.
Conflict of interest statement
Figures
References
-
- Taylor Z, Nolan CM, Blumberg HM. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54 (RR-12) - PubMed
-
- Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60(48):1650–1653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

