Impact of Rotavirus Vaccine Introduction and Postintroduction Etiology of Diarrhea Requiring Hospital Admission in Haydom, Tanzania, a Rural African Setting
- PMID: 28575304
- PMCID: PMC5850044
- DOI: 10.1093/cid/cix494
Impact of Rotavirus Vaccine Introduction and Postintroduction Etiology of Diarrhea Requiring Hospital Admission in Haydom, Tanzania, a Rural African Setting
Abstract
Background: No data are available on the etiology of diarrhea requiring hospitalization after rotavirus vaccine introduction in Africa. The monovalent rotavirus vaccine was introduced in Tanzania on 1 January 2013. We performed a vaccine impact and effectiveness study as well as a quantitative polymerase chain reaction (qPCR)-based etiology study at a rural Tanzanian hospital.
Methods: We obtained data on admissions among children <5 years to Haydom Lutheran Hospital between 1 January 2010 and 31 December 2015 and estimated the impact of vaccine introduction on all-cause diarrhea admissions. We then performed a vaccine effectiveness study using the test-negative design. Finally, we tested diarrheal specimens during 2015 by qPCR for a broad range of enteropathogens and calculated pathogen-specific attributable fractions (AFs).
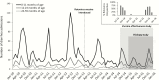
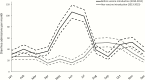
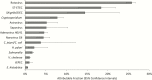
Results: Vaccine introduction was associated with a 44.9% (95% confidence interval [CI], 17.6%-97.4%) reduction in diarrhea admissions in 2015, as well as delay of the rotavirus season. The effectiveness of 2 doses of vaccine was 74.8% (95% CI, -8.2% to 94.1%) using an enzyme immunoassay-based case definition and 85.1% (95% CI, 26.5%-97.0%) using a qPCR-based case definition. Among 146 children enrolled in 2015, rotavirus remained the leading etiology of diarrhea requiring hospitalization (AF, 25.8% [95% CI, 24.4%-26.7%]), followed by heat-stable enterotoxin-producing Escherichia coli (AF, 18.4% [95% CI, 12.9%-21.9%]), Shigella/enteroinvasive E. coli (AF, 14.5% [95% CI, 10.2%-22.8%]), and Cryptosporidium (AF, 7.9% [95% CI, 6.2%-9.3%]).
Conclusions: Despite the clear impact of vaccine introduction in this setting, rotavirus remained the leading etiology of diarrhea requiring hospitalization. Further efforts to maximize vaccine coverage and improve vaccine performance in these settings are warranted.
Figures
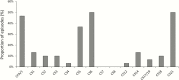
References
-
- Madhi SA, Kirsten M, Louw C et al. . Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30(suppl 1):A44–51. - PubMed
-
- Armah GE, Sow SO, Breiman RF et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

