Exclusive Enteral Nutrition Therapy in Paediatric Crohn's Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis
- PMID: 28575325
- PMCID: PMC5881686
- DOI: 10.1093/ecco-jcc/jjx060
Exclusive Enteral Nutrition Therapy in Paediatric Crohn's Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis
Abstract
Background and aims: Exclusive enteral nutrition [EEN] is recommended as a first-line induction therapy for paediatric Crohn's disease [CD] although corticosteroids [CS] are still used commonly. Our aim was to compare short- and long-term disease outcomes of paediatric CD patients initially managed with either EEN or CS.
Methods: Medical records of newly diagnosed paediatric CD patients treated with EEN or CS as induction therapy were retrospectively reviewed. To minimise selection bias inherent in observational cohort studies, propensity analysis was carried out. Data on anthropometrics, medical history, and presenting phenotype were collected at time of diagnosis [baseline]; outcomes of interest, including medication use, hospitalisation, surgical procedures, and disease progression were assessed up to 6 years following diagnosis.
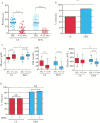
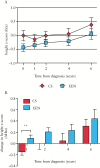
Results: Of 127 patients reviewed, a total of 111 propensity-score matched CD patients receiving EEN [n = 76] or CS [n = 35] were analysed. By 4-12 weeks of induction therapy, 86.6% of EEN-treated patients achieved remission (Paediatric Crohn's Disease Activity Index [PCDAI] ≤ 7.5) compared with 58.1% of patients in the CS-treated group [p < 0.01]. Choice of EEN over CS for induction was associated with avoidance of corticosteroids over a 6-year follow-up period. Analysis of long-term linear growth, hospitalisation, need for biologic therapy, or surgical intervention did not reveal any significant differences.
Conclusions: These findings suggest that EEN induction therapy is more effective in achieving early remission and is associated with long-term steroid avoidance without increased use of biologics or need for surgery.
Keywords: EEN; corticosteroids; propensity score.
Copyright © 2017 European Crohn’s and Colitis Organisation (ECCO). Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com
Figures

References
-
- Vernier–Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 2008;135:1106–13. - PubMed
-
- Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. - PubMed
-
- Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953–61. - PubMed
-
- Malik S, Mason A, Bakhshi A, et al. Growth in children receiving contemporary disease specific therapy for Crohn’s disease. Arch Dis Child 2012;97:698–703 - PubMed
-
- Berni Canani R, Terrin G, Borrelli O, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis 2006;38:381–7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

