Should Controls With Respiratory Symptoms Be Excluded From Case-Control Studies of Pneumonia Etiology? Reflections From the PERCH Study
- PMID: 28575354
- PMCID: PMC5447853
- DOI: 10.1093/cid/cix076
Should Controls With Respiratory Symptoms Be Excluded From Case-Control Studies of Pneumonia Etiology? Reflections From the PERCH Study
Abstract
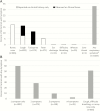
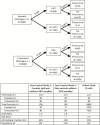
Many pneumonia etiology case-control studies exclude controls with respiratory illness from enrollment or analyses. Herein we argue that selecting controls regardless of respiratory symptoms provides the least biased estimates of pneumonia etiology. We review 3 reasons investigators may choose to exclude controls with respiratory symptoms in light of epidemiologic principles of control selection and present data from the Pneumonia Etiology Research for Child Health (PERCH) study where relevant to assess their validity. We conclude that exclusion of controls with respiratory symptoms will result in biased estimates of etiology. Randomly selected community controls, with or without respiratory symptoms, as long as they do not meet the criteria for case-defining pneumonia, are most representative of the general population from which cases arose and the least subject to selection bias.
Keywords: PERCH; control selection; pneumonia etiology; respiratory symptoms; selection bias..
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Madhi SA, Groome MJ, Zar HJ, et al. Effectiveness of pneumococcal conjugate vaccine against presumed bacterial pneumonia hospitalisation in HIV-uninfected South African children: a case-control study. Thorax 2015; 70:1149–55. - PubMed
-
- Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2 + 1 infant schedule in Quebec, Canada. Pediatr Infect Dis J 2010; 29:546–9. - PubMed
-
- Pilishvili T, Chernyshova L, Bondarenko A, et al. Evaluation of the effectiveness of Haemophilus influenzae type b conjugate vaccine introduction against radiologically-confirmed hospitalized pneumonia in young children in Ukraine. J Pediatr 2013; 163(1 suppl):S12–8. - PubMed
-
- de la Hoz F, Higuera AB, Di Fabio JL, et al. Effectiveness of Haemophilus influenzae type b vaccination against bacterial pneumonia in Colombia. Vaccine 2004; 23:36–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

