Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia
- PMID: 28575414
- PMCID: PMC5890735
- DOI: 10.1093/jac/dkx133
Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia
Abstract
Objectives: To prevent invasive fungal disease (IFD) in adult patients undergoing remission-induction chemotherapy for newly diagnosed acute lymphoblastic leukaemia (ALL).
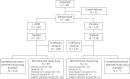
Patients and methods: In a double-blind multicentre Phase 3 study, patients received prophylactic liposomal amphotericin B (L-AMB) at 5 mg/kg intravenously or placebo twice weekly in a 2:1 random allocation during remission-induction treatment. The primary endpoint was the development of proven or probable IFD. Secondary endpoints included those focused on the safety and tolerability of prophylactic L-AMB.
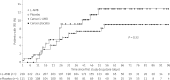
Results: Three hundred and fifty-five patients from 86 centres in Europe and South America received at least one dose of L-AMB ( n = 237) or placebo ( n = 118). Rates of proven and probable IFD assessed independently were 7.9% (18/228) in the L-AMB group and 11.7% (13/111) in the placebo group ( P = 0.24). Rates of possible IFD were 4.8% (11/228) in the L-AMB and 5.4% (6/111) in the placebo group ( P = 0.82). The remission-induction phase was a median of 22 days for both groups. Overall mortality was similar between the groups: 7.2% (17/237) for L-AMB and 6.8% (8/118) for placebo ( P = 1.00). Hypokalaemia and creatinine increase were significantly more frequent with L-AMB.
Conclusions: The IFD rate among adult patients undergoing remission-induction chemotherapy for newly diagnosed ALL was 11.7% in the placebo group, and was not significantly different in patients receiving L-AMB, suggesting that the L-AMB regimen studied is not effective as prophylaxis against IFD. The IFD rate appears higher than previously reported, warranting further investigation. Tolerability of L-AMB was what might be expected. Further studies are needed to determine the optimal antifungal strategy during remission-induction chemotherapy of ALL.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Bassan R, Hoelzer D.. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 2011; 29: 532–43. - PubMed
-
- Pagano L, Caira M, Candoni A. et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91: 1068–75. - PubMed
-
- de Pauw B, Walsh TJ, Donnelly JP. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–21. - PMC - PubMed
-
- Rüping MJ, Vehreschild JJ, Cornely OA.. Patients at high risk of invasive fungal infections: when and how to treat. Drugs 2008; 68: 1941–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

