Mutations in NKX6-2 Cause Progressive Spastic Ataxia and Hypomyelination
- PMID: 28575651
- PMCID: PMC5473715
- DOI: 10.1016/j.ajhg.2017.05.009
Mutations in NKX6-2 Cause Progressive Spastic Ataxia and Hypomyelination
Abstract
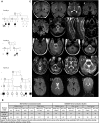
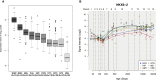
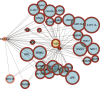
Progressive limb spasticity and cerebellar ataxia are frequently found together in clinical practice and form a heterogeneous group of degenerative disorders that are classified either as pure spastic ataxia or as complex spastic ataxia with additional neurological signs. Inheritance is either autosomal dominant or autosomal recessive. Hypomyelinating features on MRI are sometimes seen with spastic ataxia, but this is usually mild in adults and severe and life limiting in children. We report seven individuals with an early-onset spastic-ataxia phenotype. The individuals come from three families of different ethnic backgrounds. Affected members of two families had childhood onset disease with very slow progression. They are still alive in their 30s and 40s and show predominant ataxia and cerebellar atrophy features on imaging. Affected members of the third family had a similar but earlier-onset presentation associated with brain hypomyelination. Using a combination of homozygozity mapping and exome sequencing, we mapped this phenotype to deleterious nonsense or homeobox domain missense mutations in NKX6-2. NKX6-2 encodes a transcriptional repressor with early high general and late focused CNS expression. Deficiency of its mouse ortholog results in widespread hypomyelination in the brain and optic nerve, as well as in poor motor coordination in a pattern consistent with the observed human phenotype. In-silico analysis of human brain expression and network data provides evidence that NKX6-2 is involved in oligodendrocyte maturation and might act within the same pathways of genes already associated with central hypomyelination. Our results support a non-redundant developmental role of NKX6-2 in humans and imply that NKX6-2 mutations should be considered in the differential diagnosis of spastic ataxia and hypomyelination.
Keywords: NKX6-2; ataxia; genetic; leukodystrophy; recessive; spasticity.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Boespflug-Tanguy O. Inborn errors of brain myelin formation. Handb. Clin. Neurol. 2013;113:1581–1592. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

