Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome
- PMID: 28575668
- PMCID: PMC5562354
- DOI: 10.1016/j.cell.2017.05.019
Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome
Abstract
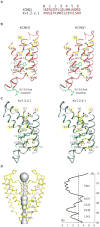
KCNQ1 is the pore-forming subunit of cardiac slow-delayed rectifier potassium (IKs) channels. Mutations in the kcnq1 gene are the leading cause of congenital long QT syndrome (LQTS). Here, we present the cryoelectron microscopy (cryo-EM) structure of a KCNQ1/calmodulin (CaM) complex. The conformation corresponds to an "uncoupled," PIP2-free state of KCNQ1, with activated voltage sensors and a closed pore. Unique structural features within the S4-S5 linker permit uncoupling of the voltage sensor from the pore in the absence of PIP2. CaM contacts the KCNQ1 voltage sensor through a specific interface involving a residue on CaM that is mutated in a form of inherited LQTS. Using an electrophysiological assay, we find that this mutation on CaM shifts the KCNQ1 voltage-activation curve. This study describes one physiological form of KCNQ1, depolarized voltage sensors with a closed pore in the absence of PIP2, and reveals a regulatory interaction between CaM and KCNQ1 that may explain CaM-mediated LQTS.
Keywords: CaM; KCNQ1; PIP2; cryo-EM; ion channel structure; long QT syndrome.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

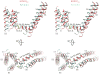


References
-
- Abbott GW. Biology of the KCNQ1 Potassium Channel. New Journal of Science. 2014;2014:1–26.
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Boczek NJ, Gomez-Hurtado N, Ye D, Calvert ML, Tester DJ, Kryshtal DO, Hwang HS, Johnson CN, Chazin WJ, Loporcaro CG, et al. Spectrum and Prevalence of CALM1-, CALM2-, and CALM3-Encoded Calmodulin Variants in Long QT Syndrome and Functional Characterization of a Novel Long QT Syndrome-Associated Calmodulin Missense Variant, E141G. Circ Cardiovasc Genet. 2016;9:136–146. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

