Structural Basis for Mitotic Centrosome Assembly in Flies
- PMID: 28575671
- PMCID: PMC5457487
- DOI: 10.1016/j.cell.2017.05.030
Structural Basis for Mitotic Centrosome Assembly in Flies
Abstract
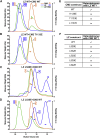
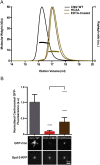
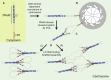
In flies, Centrosomin (Cnn) forms a phosphorylation-dependent scaffold that recruits proteins to the mitotic centrosome, but how Cnn assembles into a scaffold is unclear. We show that scaffold assembly requires conserved leucine zipper (LZ) and Cnn-motif 2 (CM2) domains that co-assemble into a 2:2 complex in vitro. We solve the crystal structure of the LZ:CM2 complex, revealing that both proteins form helical dimers that assemble into an unusual tetramer. A slightly longer version of the LZ can form micron-scale structures with CM2, whose assembly is stimulated by Plk1 phosphorylation in vitro. Mutating individual residues that perturb LZ:CM2 tetramer assembly perturbs the formation of these micron-scale assemblies in vitro and Cnn-scaffold assembly in vivo. Thus, Cnn molecules have an intrinsic ability to form large, LZ:CM2-interaction-dependent assemblies that are critical for mitotic centrosome assembly. These studies provide the first atomic insight into a molecular interaction required for mitotic centrosome assembly.
Keywords: Centrosomin; Cnn; PCM; Plk1; centriole; centrosome; mitosis.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures










Comment in
-
Centrosome Biology: Polymer-Based Centrosome Maturation.Curr Biol. 2017 Sep 11;27(17):R836-R839. doi: 10.1016/j.cub.2017.07.036. Curr Biol. 2017. PMID: 28898644
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

