Biomaterials for skeletal muscle tissue engineering
- PMID: 28575733
- PMCID: PMC5617779
- DOI: 10.1016/j.copbio.2017.05.003
Biomaterials for skeletal muscle tissue engineering
Abstract
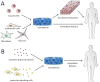
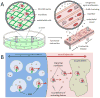
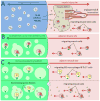
Although skeletal muscle can naturally regenerate in response to minor injuries, more severe damage and myopathies can cause irreversible loss of muscle mass and function. Cell therapies, while promising, have not yet demonstrated consistent benefit, likely due to poor survival of delivered cells. Biomaterials can improve muscle regeneration by presenting chemical and physical cues to muscle cells that mimic the natural cascade of regeneration. This brief review describes strategies for muscle repair utilizing biomaterials that can provide signals to either transplanted or host muscle cells. These strategies range from approaches that utilize biomaterials alone to those that combine biomaterials with exogenous growth factors, ex vivo cultured cells, and extensive culture time.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
References
-
- Chuang DC-C. Free tissue transfer for the treatment of facial paralysis. Facial Plastic Surgery. 2008;24:194–203. - PubMed
-
- Palmieri B, Tremblay JP, Daniele L. Past, present and future of myoblast transplantation in the treatment of Duchenne muscular dystrophy. Pediatric transplantation. 2010;14:813–819. - PubMed
-
- Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries biology and treatment. The American journal of sports medicine. 2005;33:745–764. - PubMed
-
- Fishman JM, Tyraskis A, Maghsoudlou P, Urbani L, Totonelli G, Birchall MA, De Coppi P. Skeletal muscle tissue engineering: which cell to use? Tissue Engineering Part B: Reviews. 2013;19:503–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

