Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis
- PMID: 28576166
- PMCID: PMC5545121
- DOI: 10.1016/j.cpet.2017.02.001
Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis
Abstract
Increased human epidermal growth factor receptor 2 (HER2) expression is a hallmark of aggressive breast cancer. Imaging modalities have the potential to diagnose HER2-positive breast cancer and detect distant metastases. The heterogeneity of HER2 expression between primary and metastatic disease sites limits the value of tumor biopsies. Molecular imaging is a noninvasive tool to assess HER2-positive primary lesions and metastases. Radiolabeled antibodies, antibody fragments, and affibody molecules devise a reliable and quantitative method for detecting HER2-positive cancer using PET. HER2-targeted PET imaging is a valuable clinical tool with respect to both the care and maintenance of patients with breast cancer.
Keywords: Breast cancer; CT; HER2; Imaging; Metastasis; PET; Radiotracers; SPECT.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
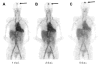
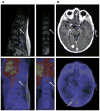

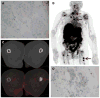
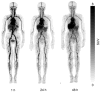
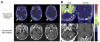
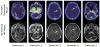
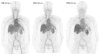



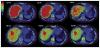



References
-
- Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. - PubMed
-
- Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

