α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration
- PMID: 28576704
- PMCID: PMC5701756
- DOI: 10.1016/j.nbd.2017.05.014
α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration
Erratum in
-
Corrigendum to "α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration" [Neurobiol. Dis., Volume 105 (2017) Article 84, 98].Neurobiol Dis. 2021 Nov;159:105506. doi: 10.1016/j.nbd.2021.105506. Epub 2021 Sep 24. Neurobiol Dis. 2021. PMID: 34565675 No abstract available.
Abstract
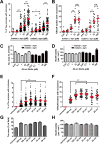
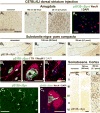
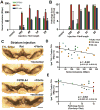
Proteinaceous inclusions in neurons, composed primarily of α-synuclein, define the pathology in several neurodegenerative disorders. Neurons can internalize α-synuclein fibrils that can seed new inclusions from endogenously expressed α-synuclein. The factors contributing to the spread of pathology and subsequent neurodegeneration are not fully understood, and different compositions and concentrations of fibrils have been used in different hosts. Here, we systematically vary the concentration and length of well-characterized α-synuclein fibrils and determine their relative ability to induce inclusions and neurodegeneration in different hosts (primary neurons, C57BL/6J and C3H/HeJ mice, and Sprague Dawley rats). Using dynamic-light scattering profiles and other measurements to determine fibril length and concentration, we find that femptomolar concentrations of fibrils are sufficient to induce robust inclusions in primary neurons. However, a narrow and non-linear dynamic range characterizes fibril-mediated inclusion induction in axons and the soma. In mice, the C3H/HeJ strain is more sensitive to fibril exposures than C57BL/6J counterparts, with more inclusions and dopaminergic neurodegeneration. In rats, injection of fibrils into the substantia nigra pars compacta (SNpc) results in similar inclusion spread and dopaminergic neurodegeneration as injection of the fibrils into the dorsal striatum, with prominent inclusion spread to the amygdala and several other brain areas. Inclusion spread, particularly from the SNpc to the striatum, positively correlates with dopaminergic neurodegeneration. These results define biophysical characteristics of α-synuclein fibrils that induce inclusions and neurodegeneration both in vitro and in vivo, and suggest that inclusion spread in the brain may be promoted by a loss of neurons.
Keywords: Aggregation; NACP; Parkinson disease; Prion; SNCA.
Copyright © 2017. Published by Elsevier Inc.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

