The Syk Tyrosine Kinase Is Required for Skin Inflammation in an In Vivo Mouse Model of Epidermolysis Bullosa Acquisita
- PMID: 28576735
- PMCID: PMC5624865
- DOI: 10.1016/j.jid.2017.05.017
The Syk Tyrosine Kinase Is Required for Skin Inflammation in an In Vivo Mouse Model of Epidermolysis Bullosa Acquisita
Abstract
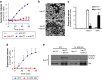
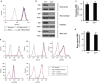
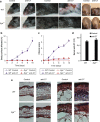
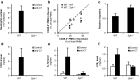
The inflammatory form of epidermolysis bullosa acquisita is caused by autoantibodies against type VII collagen (C7), a component of the dermal-epidermal junction. We have previously shown that myeloid Src family kinases mediate skin inflammation triggered by anti-C7 antibodies. Here we identify the Syk tyrosine kinase as a critical component of autoantibody-induced skin inflammation downstream of Src family kinases. Immobilized C7-anti-C7 immune complexes triggered neutrophil activation and Syk phosphorylation in a Src family kinase-dependent manner. Bone marrow chimeric mice lacking Syk in their hematopoietic compartment were completely protected from skin inflammation triggered by anti-C7 antibodies despite normal circulating anti-C7 levels. Syk deficiency abrogated the accumulation of CXCL2, IL-1β, and leukotriene B4 at the site of inflammation and resulted in defective in vivo neutrophil recruitment. Syk-/- neutrophils had a normal intrinsic migratory capacity but failed to release CXCL2 or leukotriene B4 upon activation by immobilized C7-anti-C7 immune complexes, indicating a role for Syk in the amplification of the inflammation process. These results identify Syk as a critical component of skin inflammation in a mouse model of epidermolysis bullosa acquisita and as a potential therapeutic target in epidermolysis bullosa acquisita and other mechanistically related inflammatory skin diseases such as bullous pemphigoid.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Whole-Genome Expression Profiling in Skin Reveals SYK As a Key Regulator of Inflammation in Experimental Epidermolysis Bullosa Acquisita.Front Immunol. 2018 Feb 15;9:249. doi: 10.3389/fimmu.2018.00249. eCollection 2018. Front Immunol. 2018. PMID: 29497423 Free PMC article.
-
Neutrophil-Specific Syk Expression Is Crucial for Skin Disease in Experimental Epidermolysis Bullosa Acquisita.J Invest Dermatol. 2023 Jul;143(7):1147-1156. doi: 10.1016/j.jid.2022.12.016. Epub 2023 Jan 11. J Invest Dermatol. 2023. PMID: 36641133
-
The Leukotriene B4 and its Receptor BLT1 Act as Critical Drivers of Neutrophil Recruitment in Murine Bullous Pemphigoid-Like Epidermolysis Bullosa Acquisita.J Invest Dermatol. 2017 May;137(5):1104-1113. doi: 10.1016/j.jid.2016.12.021. Epub 2017 Jan 17. J Invest Dermatol. 2017. PMID: 28108297
-
Epidermolysis bullosa acquisita: what's new?J Dermatol. 2010 Mar;37(3):220-30. doi: 10.1111/j.1346-8138.2009.00799.x. J Dermatol. 2010. PMID: 20507385 Review.
-
Immune mechanism-targeted treatment of experimental epidermolysis bullosa acquisita.Expert Rev Clin Immunol. 2015;11(12):1365-78. doi: 10.1586/1744666X.2015.1085801. Epub 2015 Oct 15. Expert Rev Clin Immunol. 2015. PMID: 26471717 Review.
Cited by
-
Editorial: Autoantibodies.Front Immunol. 2019 Apr 2;10:484. doi: 10.3389/fimmu.2019.00484. eCollection 2019. Front Immunol. 2019. PMID: 31001243 Free PMC article. No abstract available.
-
Hematopoietic or Osteoclast-Specific Deletion of Syk Leads to Increased Bone Mass in Experimental Mice.Front Immunol. 2019 Apr 30;10:937. doi: 10.3389/fimmu.2019.00937. eCollection 2019. Front Immunol. 2019. PMID: 31134061 Free PMC article.
-
Whole-Genome Expression Profiling in Skin Reveals SYK As a Key Regulator of Inflammation in Experimental Epidermolysis Bullosa Acquisita.Front Immunol. 2018 Feb 15;9:249. doi: 10.3389/fimmu.2018.00249. eCollection 2018. Front Immunol. 2018. PMID: 29497423 Free PMC article.
-
Myeloid Src-family kinases are critical for neutrophil-mediated autoinflammation in gout and motheaten models.J Exp Med. 2023 Jul 3;220(7):e20221010. doi: 10.1084/jem.20221010. Epub 2023 Apr 19. J Exp Med. 2023. PMID: 37074415 Free PMC article.
-
Lineage-Specific Analysis of Syk Function in Autoantibody-Induced Arthritis.Front Immunol. 2018 Mar 19;9:555. doi: 10.3389/fimmu.2018.00555. eCollection 2018. Front Immunol. 2018. PMID: 29616043 Free PMC article.
References
-
- Baum S., Sakka N., Artsi O., Trau H., Barzilai A. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482–489. - PubMed
-
- Berton G., Mócsai A., Lowell C.A. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. - PubMed
-
- Chiriac M.T., Roesler J., Sindrilaru A., Scharffetter-Kochanek K., Zillikens D., Sitaru C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J Pathol. 2007;212:56–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

