Incompatibility between mitochondrial and nuclear genomes during oogenesis results in ovarian failure and embryonic lethality
- PMID: 28576772
- PMCID: PMC5536873
- DOI: 10.1242/dev.151951
Incompatibility between mitochondrial and nuclear genomes during oogenesis results in ovarian failure and embryonic lethality
Abstract
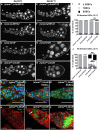
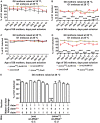
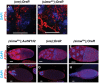
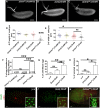
Mitochondrial dysfunction can cause female infertility. An important unresolved issue is the extent to which incompatibility between mitochondrial and nuclear genomes contributes to female infertility. It has previously been shown that a mitochondrial haplotype from D. simulans (simw501 ) is incompatible with a nuclear genome from the D. melanogaster strain Oregon-R (OreR), resulting in impaired development, which was enhanced at higher temperature. This mito-nuclear incompatibility is between alleles of the nuclear-encoded mitochondrial tyrosyl-tRNA synthetase (Aatm) and the mitochondrial-encoded tyrosyl-tRNA that it aminoacylates. Here, we show that this mito-nuclear incompatibility causes a severe temperature-sensitive female infertility. The OreR nuclear genome contributed to death of ovarian germline stem cells and reduced egg production, which was further enhanced by the incompatibility with simw501 mitochondria. Mito-nuclear incompatibility also resulted in aberrant egg morphology and a maternal-effect on embryonic chromosome segregation and survival, which was completely dependent on the temperature and mito-nuclear genotype of the mother. Our findings show that maternal mito-nuclear incompatibility during Drosophila oogenesis has severe consequences for egg production and embryonic survival, with important broader relevance to human female infertility and mitochondrial replacement therapy.
Keywords: Drosophila; Embryogenesis; Mitochondria; Mitochondrial-nuclear incompatibility; Oogenesis; Stem cell.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

