BRAF Mutations as Predictive Biomarker for Response to Anti-EGFR Monoclonal Antibodies
- PMID: 28576857
- PMCID: PMC5507642
- DOI: 10.1634/theoncologist.2017-0031
BRAF Mutations as Predictive Biomarker for Response to Anti-EGFR Monoclonal Antibodies
Abstract
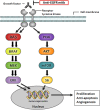
Recently, the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) recommended that patients with epidermal growth factor receptor (EGFR)-expressing metastatic colorectal cancer could be treated with anti-EGFR monoclonal antibodies (mAbs) cetuximab and panitumumab only in absence of Rat-Sarcoma (RAS) mutations. In addition to the previously established biomarker Kirsten rat sarcoma viral oncogene homolog (KRAS) exon 2, cumulative evidence also shows that patients whose tumors harbor KRAS exons 3 or 4 and neuroblastoma rat-sarcoma viral oncogene homolog (NRAS) exons 2, 3, and 4 mutations are found unlikely to benefit from anti-EGFR treatment.In line with the resistance of RAS mutated (mt) tumors, treatment response in BRAFmt tumors may also be altered given their important role in the EGFR signaling pathway. However, BRAF is not recommended as predictive biomarker yet because the evidence for the impact of BRAF mutations on treatment outcome is considered insufficient.This article summarizes the evidence for the impact of BRAF mutations on treatment outcome of anti-EGFR mAbs. Based on a review of literature, eight meta-analyses were included that consistently show that patients with BRAF mutations have a lack of treatment benefit of anti-EGFR mAbs. After discussing the quality and quantity of available evidence, we conclude that evidence is stronger than suggested by ESMO and ASCO. Additionally, we highlight that the quality of evidence for BRAF is even higher than for extended RAS as a biomarker. We therefore advise ESMO and ASCO to reconsider BRAF status as a predictive biomarker for response.
Implications for practice: In metastatic colorectal cancer (mCRC), therapy with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab is indicated in absence of RAS mutations. Cumulative evidence shows that patients with BRAF mutations, who comprise 10% of the mCRC population, do not benefit from anti-EGFR-antibody treatment. Although guidelines state that evidence for BRAF as a predictive marker is insufficient, we highlight that the quality and quantity of evidence is higher than suggested. We therefore encourage the use of BRAF as a predictive marker in order to exclude patients from therapy for whom limited treatment benefit is expected.
Keywords: Anti‐epidermal growth factor receptor; BRAF; Cetuximab; Panitumumab; Predictive biomarker; RAS.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?Genet Med. 2013 Jul;15(7):517-27. doi: 10.1038/gim.2012.184. Epub 2013 Feb 21. Genet Med. 2013. PMID: 23429431
-
The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis.Acta Oncol. 2014 Jul;53(7):852-64. doi: 10.3109/0284186X.2014.895036. Epub 2014 Mar 25. Acta Oncol. 2014. PMID: 24666267
-
Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials.Ann Oncol. 2015 Jan;26(1):13-21. doi: 10.1093/annonc/mdu378. Epub 2014 Aug 12. Ann Oncol. 2015. PMID: 25115304
-
Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer.J Natl Cancer Inst. 2009 Oct 7;101(19):1308-24. doi: 10.1093/jnci/djp280. Epub 2009 Sep 8. J Natl Cancer Inst. 2009. PMID: 19738166 Free PMC article. Review.
-
Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer.Int J Clin Oncol. 2013 Aug;18(4):670-7. doi: 10.1007/s10147-012-0422-8. Epub 2012 May 26. Int J Clin Oncol. 2013. PMID: 22638623
Cited by
-
Genetics of rectal cancer and novel therapies: primer for radiologists.Abdom Radiol (NY). 2019 Nov;44(11):3743-3750. doi: 10.1007/s00261-019-02051-x. Abdom Radiol (NY). 2019. PMID: 31073721 Free PMC article. Review.
-
Colorectal cancer genomics and designing rational trials.Ann Transl Med. 2018 May;6(9):159. doi: 10.21037/atm.2018.03.27. Ann Transl Med. 2018. PMID: 29911107 Free PMC article. Review.
-
Prognostic Value of BRAF and KRAS Mutation in Relation to Colorectal Cancer Survival in Iranian Patients: Correlated to Microsatellite Instability.J Gastrointest Cancer. 2020 Mar;51(1):53-62. doi: 10.1007/s12029-019-00201-4. J Gastrointest Cancer. 2020. PMID: 30635874
-
The impact of panitumumab treatment on survival and quality of life in patients with RAS wild-type metastatic colorectal cancer.Cancer Manag Res. 2019 Jun 28;11:5911-5924. doi: 10.2147/CMAR.S186042. eCollection 2019. Cancer Manag Res. 2019. PMID: 31388315 Free PMC article.
-
BRAF Mutation in Colorectal Cancers: From Prognostic Marker to Targetable Mutation.Cancers (Basel). 2020 Nov 3;12(11):3236. doi: 10.3390/cancers12113236. Cancers (Basel). 2020. PMID: 33152998 Free PMC article. Review.
References
-
- Allegra CJ, Rumble RB, Hamilton SR et al. Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti‐epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol 2016;34:179–185. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422. - PubMed
-
- Welcome Trust Sanger Institute. Cosmic: Cancer Browser. Available at https://cancer.sanger.ac.uk/cosmic/browse/tissue#sn=large_intestine&ss= .... Accessed August 18, 2016.
-
- De Roock W, Claes B, Bernasconi D et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy‐refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol 2010;11:753–762. - PubMed
-
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous