Origin of a rapidly evolving homeostatic control system programming testis function
- PMID: 28576872
- PMCID: PMC5529123
- DOI: 10.1530/JOE-17-0250
Origin of a rapidly evolving homeostatic control system programming testis function
Abstract
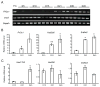
Mammals share common strategies for regulating reproduction, including a conserved hypothalamic-pituitary-gonadal axis; yet, individual species exhibit differences in reproductive performance. In this report, we describe the discovery of a species-restricted homeostatic control system programming testis growth and function. Prl3c1 is a member of the prolactin gene family and its protein product (PLP-J) was discovered as a uterine cytokine contributing to the establishment of pregnancy. We utilized mouse mutagenesis of Prl3c1 and revealed its involvement in the regulation of the male reproductive axis. The Prl3c1-null male reproductive phenotype was characterized by testiculomegaly and hyperandrogenism. The larger testes in the Prl3c1-null mice were associated with an expansion of the Leydig cell compartment. Prl3c1 locus is a template for two transcripts (Prl3c1-v1 and Prl3c1-v2) expressed in a tissue-specific pattern. Prl3c1-v1 is expressed in uterine decidua, while Prl3c1-v2 is expressed in Leydig cells of the testis. 5'RACE, chromatin immunoprecipitation and DNA methylation analyses were used to define cell-specific promoter usage and alternative transcript expression. We examined the Prl3c1 locus in five murid rodents and showed that the testicular transcript and encoded protein are the result of a recent retrotransposition event at the Mus musculus Prl3c1 locus. Prl3c1-v1 encodes PLP-J V1 and Prl3c1-v2 encodes PLP-J V2. Each protein exhibits distinct intracellular targeting and actions. PLP-J V2 possesses Leydig cell-static actions consistent with the Prl3c1-null testicular phenotype. Analysis of the biology of the Prl3c1 gene has provided insight into a previously unappreciated homeostatic setpoint control system programming testicular growth and function.
Keywords: Leydig cells; prolactin family; testis; transposable elements.
© 2017 Society for Endocrinology.
Conflict of interest statement
Figures




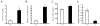



References
-
- Achermann JC, Jameson JL. Fertility and infertility: genetic contributions from the hypothalamic-pituitary-gonadal axis. Molecular Endocrinology. 1999;13:812–818. - PubMed
-
- Alam SMK, Konno T, Dai G, Lu L, Wang D, Dunmore JH, Godwin AR, Soares MJ. A uterine decidual cell cytokine ensures pregnancy-dependent adaptations to a physiological stressor. Development. 2007;134:407–415. - PubMed
-
- Amory JD, Bremner W. Endocrine regulation of testicular function in men: implications for contraceptive development. Molecular and Cellular Endocrinology. 2001;182:175–179. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

