Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine
- PMID: 28576876
- PMCID: PMC5942867
- DOI: 10.1182/blood-2016-10-745588
Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine
Abstract
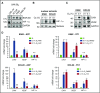
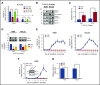
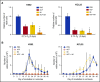
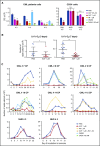
Chronic myeloid leukemia (CML) is a hematopoietic stem cell (HSC)-driven neoplasia characterized by expression of the constitutively active tyrosine kinase BCR/Abl. CML therapy based on tyrosine kinase inhibitors (TKIs) is highly effective in inducing remission but not in targeting leukemia stem cells (LSCs), which sustain minimal residual disease and are responsible for CML relapse following discontinuation of treatment. The identification of molecules capable of targeting LSCs appears therefore of primary importance to aim at CML eradication. LSCs home in bone marrow areas at low oxygen tension, where HSCs are physiologically hosted. This study addresses the effects of pharmacological inhibition of hypoxia-inducible factor-1 (HIF-1), a critical regulator of LSC survival, on the maintenance of CML stem cell potential. We found that the HIF-1 inhibitor acriflavine (ACF) decreased survival and growth of CML cells. These effects were paralleled by decreased expression of c-Myc and stemness-related genes. Using different in vitro stem cell assays, we showed that ACF, but not TKIs, targets the stem cell potential of CML cells, including primary cells explanted from 12 CML patients. Moreover, in a murine CML model, ACF decreased leukemia development and reduced LSC maintenance. Importantly, ACF exhibited significantly less-severe effects on non-CML hematopoietic cells in vitro and in vivo. Thus, we propose ACF, a US Food and Drug Administration (FDA)-approved drug for nononcological use in humans, as a novel therapeutic approach to prevent CML relapse and, in combination with TKIs, enhance induction of remission.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
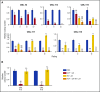
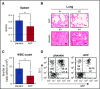
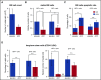
References
-
- Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038-1042. - PubMed
-
- Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319-325. - PubMed
-
- Giuntoli S, Rovida E, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Hypoxia suppresses BCR/Abl and selects imatinib-insensitive progenitors within clonal CML populations. Leukemia. 2006;20(7):1291-1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

