Cholesteryl esters of ω-(O-acyl)-hydroxy fatty acids in vernix caseosa
- PMID: 28576934
- PMCID: PMC5538280
- DOI: 10.1194/jlr.M075333
Cholesteryl esters of ω-(O-acyl)-hydroxy fatty acids in vernix caseosa
Abstract
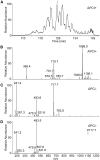
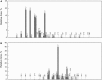
Cholesteryl esters of ω-(O-acyl)-hydroxy FAs (Chl-ωOAHFAs) were identified for the first time in vernix caseosa and characterized using chromatography and MS. Chl-ωOAHFAs were isolated using adsorption chromatography on silica gel and magnesium hydroxide. Their general structure was established using high-resolution and tandem MS of intact lipids, and products of their transesterification and derivatizations. Individual molecular species were characterized using nonaqueous reversed-phase HPLC coupled to atmospheric pressure chemical ionization. The analytes were detected as protonated molecules, and their structures were elucidated in the negative ion mode using controlled thermal decomposition and data-dependent fragmentation. About three hundred molecular species of Chl-ωOAHFAs were identified in this way. The most abundant Chl-ωOAHFAs contained 32:1 ω-hydroxy FA (ω-HFA) and 14:0, 15:0, 16:0, 16:1, and 18:1 FAs. The double bond in the 32:1 ω-HFA was in the n-7 and n-9 positions. Chl-ωOAHFAs are estimated to account for approximately 1-2% of vernix caseosa lipids.
Keywords: cholesterol; lipidomics; mass spectrometry; neutral lipids; skin lipids.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Haubrich K. A. 2003. Role of vernix caseosa in the neonate: potential application in the adult population. AACN Clin. Issues. 14: 457–464. - PubMed
-
- Hoath S. B., Pickens W. L., and Visscher M. O.. 2006. The biology of vernix caseosa. Int. J. Cosmet. Sci. 28: 319–333. - PubMed
-
- Schmid R. 1939. Notizen zur Kenntnis der Vernix caseosa. Arch. Gynakol. 168: 445–450.
-
- Rissmann R., Groenink H. W. W., Weerheim A. M., Hoath S. B., Ponec M., and Bouwstra J. A.. 2006. New insights into ultrastructure, lipid composition and organization of vernix caseosa. J. Invest. Dermatol. 126: 1823–1833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

