Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity
- PMID: 28576970
- PMCID: PMC5536914
- DOI: 10.1242/jcs.192781
Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity
Abstract
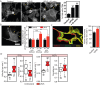
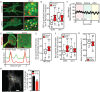
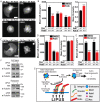
Low-intensity pulsed ultrasound (LIPUS) is a therapy used clinically to promote healing. Using live-cell imaging we show that LIPUS stimulation, acting through integrin-mediated cell-matrix adhesions, rapidly induces Rac1 activation associated with dramatic actin cytoskeleton rearrangements. Our study demonstrates that the mechanosensitive focal adhesion (FA) protein vinculin, and both focal adhesion kinase (FAK, also known as PTK2) and Rab5 (both the Rab5a and Rab5b isoforms) have key roles in regulating these effects. Inhibiting the link of vinculin to the actin-cytoskeleton abolished LIPUS sensing. We show that this vinculin-mediated link was not only critical for Rac1 induction and actin rearrangements, but was also important for the induction of a Rab5-dependent increase in the number of early endosomes. Expression of dominant-negative Rab5, or inhibition of endocytosis with dynasore, also blocked LIPUS-induced Rac1 signalling events. Taken together, our data show that LIPUS is sensed by cell matrix adhesions through vinculin, which in turn modulates a Rab5-Rac1 pathway to control ultrasound-mediated endocytosis and cell motility. Finally, we demonstrate that a similar FAK-Rab5-Rac1 pathway acts to control cell spreading upon fibronectin.
Keywords: Endocytosis; LIPUS; Migration; Rac1; Vinculin.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsA.H. is an employee of Bioventus that manufactures and sells a LIPUS device known as EXOGEN.
Figures





Similar articles
-
Rab5 isoforms orchestrate a "division of labor" in the endocytic network; Rab5C modulates Rac-mediated cell motility.PLoS One. 2014 Feb 28;9(2):e90384. doi: 10.1371/journal.pone.0090384. eCollection 2014. PLoS One. 2014. PMID: 24587345 Free PMC article.
-
Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly.J Biol Chem. 2006 Jul 7;281(27):18652-9. doi: 10.1074/jbc.M603508200. Epub 2006 May 11. J Biol Chem. 2006. PMID: 16698790
-
Induction of adhesion-dependent signals using low-intensity ultrasound.J Vis Exp. 2012 May 8;(63):e4024. doi: 10.3791/4024. J Vis Exp. 2012. PMID: 22588522 Free PMC article.
-
Integrin-mediated cell adhesion: the cytoskeletal connection.Biochem Soc Symp. 1999;65:79-99. Biochem Soc Symp. 1999. PMID: 10320934 Review.
-
Mechanosensitive components of integrin adhesions: Role of vinculin.Exp Cell Res. 2016 Apr 10;343(1):21-27. doi: 10.1016/j.yexcr.2015.11.017. Epub 2015 Nov 24. Exp Cell Res. 2016. PMID: 26607713 Free PMC article. Review.
Cited by
-
Sonomechanobiology: Vibrational stimulation of cells and its therapeutic implications.Biophys Rev (Melville). 2023 Apr 21;4(2):021301. doi: 10.1063/5.0127122. eCollection 2023 Jun. Biophys Rev (Melville). 2023. PMID: 38504927 Free PMC article. Review.
-
The role of biophysical cues and their modulated exosomes in dental diseases: from mechanism to therapy.Stem Cell Res Ther. 2024 Oct 19;15(1):373. doi: 10.1186/s13287-024-03990-z. Stem Cell Res Ther. 2024. PMID: 39427216 Free PMC article. Review.
-
Optimal low-intensity pulsed ultrasound stimulation for promoting anti-inflammatory effects in macrophages.APL Bioeng. 2023 Mar 22;7(1):016114. doi: 10.1063/5.0137881. eCollection 2023 Mar. APL Bioeng. 2023. PMID: 36968453 Free PMC article.
-
Integrin trafficking in cells and tissues.Nat Cell Biol. 2019 Feb;21(2):122-132. doi: 10.1038/s41556-018-0223-z. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602723 Free PMC article. Review.
-
Acoustic technologies for the orchestration of cellular functions for therapeutic applications.Sci Adv. 2025 Jul 18;11(29):eadu4759. doi: 10.1126/sciadv.adu4759. Epub 2025 Jul 18. Sci Adv. 2025. PMID: 40680134 Free PMC article. Review.
References
-
- Argadine H., Kinnick R., Bolander M. and Greenleaf J. F. (2005). 1 kHz low power sound stimulates ATDC5 chondrocytes. Proc. IEEE Ultrason Symp. 2, 996-998. 10.1109/ultsym.2005.1603018 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

