Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy
- PMID: 28577085
- PMCID: PMC5700458
- DOI: 10.1007/s00262-017-2019-6
Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy
Abstract
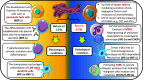
The endoplasmic reticulum (ER) stress is a cellular process that occurs as a consequence of several stress circumstances, such as the accumulation of unfolded proteins in the lumen of the ER or distinct insults that disturb the ER normal function. Different conditions in the tumor microenvironment (TME), including hypoxia, nutrient deprivation, and the elevated production of reactive oxygen and nitrogen species destabilize the loading and dispatching of the newly synthesized proteins, triggering ER stress in cancer cells and tumor-infiltrating leukocytes. In order to cope with TME-induced ER stress, tumor and stromal cells initiate an adaptive response process that aims to resolve ER stress and to restore cellular homeostasis, which is referred as the unfolded protein responses (UPR). Paradoxically, the UPR can also induce cell death under severe and/or permanent ER stress. The UPR is started through three mediators, the activation of the inositol-requiring enzyme-1α, the pancreatic ER kinase-like ER kinase, and the activating transcription factor 6. In this minireview, we will discuss the pro- and anti-tumorigenic role of the UPR in cancer cells. In addition, we will describe the effects of the TME-induced ER stress in the immunosuppressive activity of tumor-infiltrating myeloid cells. Also, we will review the results of emerging therapeutic interventions that target ER stress and the UPR mediators in cancer. We postulate that the inhibition of ER stress or the UPR-related elements could represent a significant approach to increase the efficacy of various forms of cancer immunotherapy.
Keywords: Endoplasmic reticulum stress; Myeloid-derived suppressor cells; Regulatory myeloid suppressor cells; T cells; Tumor microenvironment; Unfolded protein responses.
Conflict of interest statement
The authors do not have conflict of interests to disclose.
Figures

Similar articles
-
Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer.J Immunother Cancer. 2017 Jan 17;5:5. doi: 10.1186/s40425-016-0203-4. eCollection 2017. J Immunother Cancer. 2017. PMID: 28105371 Free PMC article. Review.
-
Unraveling UPR-mediated intercellular crosstalk: Implications for immunotherapy resistance mechanisms.Cancer Lett. 2025 May 1;617:217613. doi: 10.1016/j.canlet.2025.217613. Epub 2025 Mar 5. Cancer Lett. 2025. PMID: 40054654 Review.
-
Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer.Cell. 2017 Feb 9;168(4):692-706. doi: 10.1016/j.cell.2016.12.004. Cell. 2017. PMID: 28187289 Free PMC article. Review.
-
Emerging Role of the Unfolded Protein Response in Tumor Immunosurveillance.Trends Cancer. 2017 Jul;3(7):491-505. doi: 10.1016/j.trecan.2017.05.005. Epub 2017 Jul 6. Trends Cancer. 2017. PMID: 28718404 Review.
-
Endoplasmic reticulum stress signals in the tumour and its microenvironment.Nat Rev Cancer. 2021 Feb;21(2):71-88. doi: 10.1038/s41568-020-00312-2. Epub 2020 Nov 19. Nat Rev Cancer. 2021. PMID: 33214692 Free PMC article. Review.
Cited by
-
YAP promotes gastric cancer cell survival and migration/invasion via the ERK/endoplasmic reticulum stress pathway.Oncol Lett. 2019 Dec;18(6):6752-6758. doi: 10.3892/ol.2019.11049. Epub 2019 Nov 4. Oncol Lett. 2019. PMID: 31807184 Free PMC article.
-
Ping-Pong-Tumor and Host in Pancreatic Cancer Progression.Front Oncol. 2019 Dec 16;9:1359. doi: 10.3389/fonc.2019.01359. eCollection 2019. Front Oncol. 2019. PMID: 31921628 Free PMC article. Review.
-
Roles of HMGB1 in regulating myeloid-derived suppressor cells in the tumor microenvironment.Biomark Res. 2020 Jun 16;8:21. doi: 10.1186/s40364-020-00201-8. eCollection 2020. Biomark Res. 2020. PMID: 32551121 Free PMC article. Review.
-
Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia.Int J Mol Sci. 2021 May 24;22(11):5555. doi: 10.3390/ijms22115555. Int J Mol Sci. 2021. PMID: 34074034 Free PMC article.
-
ER stress-related mRNA-lncRNA co-expression gene signature predicts the prognosis and immune implications of esophageal cancer.Am J Transl Res. 2022 Nov 15;14(11):8064-8084. eCollection 2022. Am J Transl Res. 2022. PMID: 36505280 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

