Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy
- PMID: 28577568
- PMCID: PMC5457546
- DOI: 10.1186/s12931-017-0600-3
Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy
Abstract
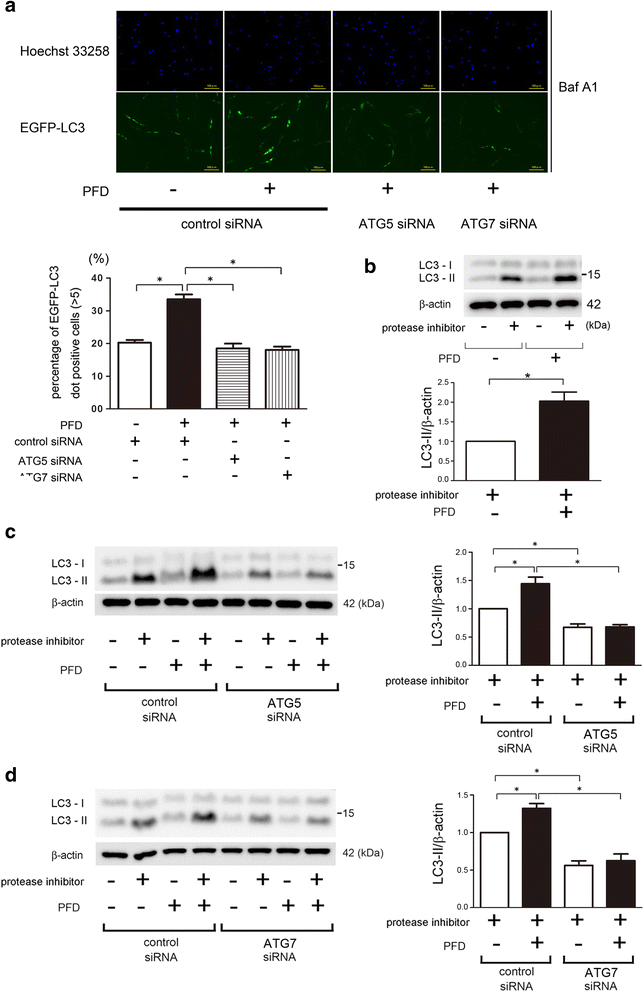
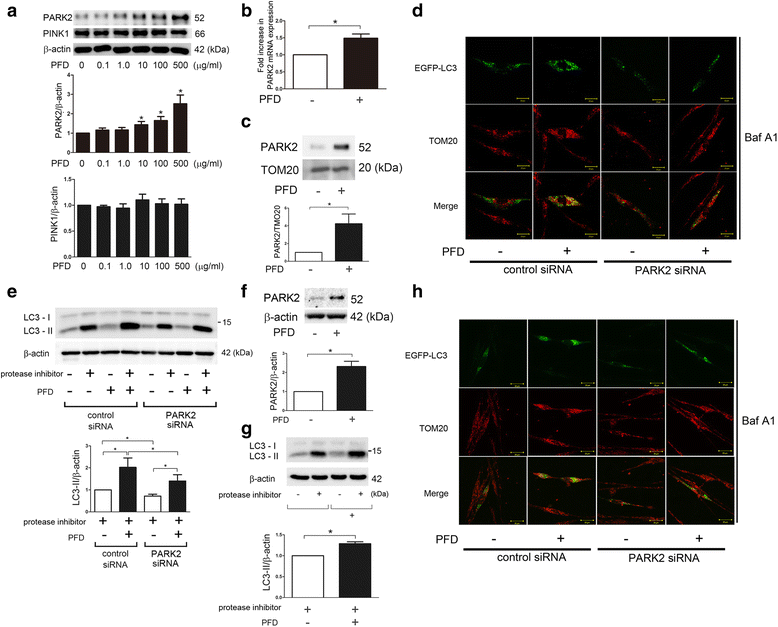
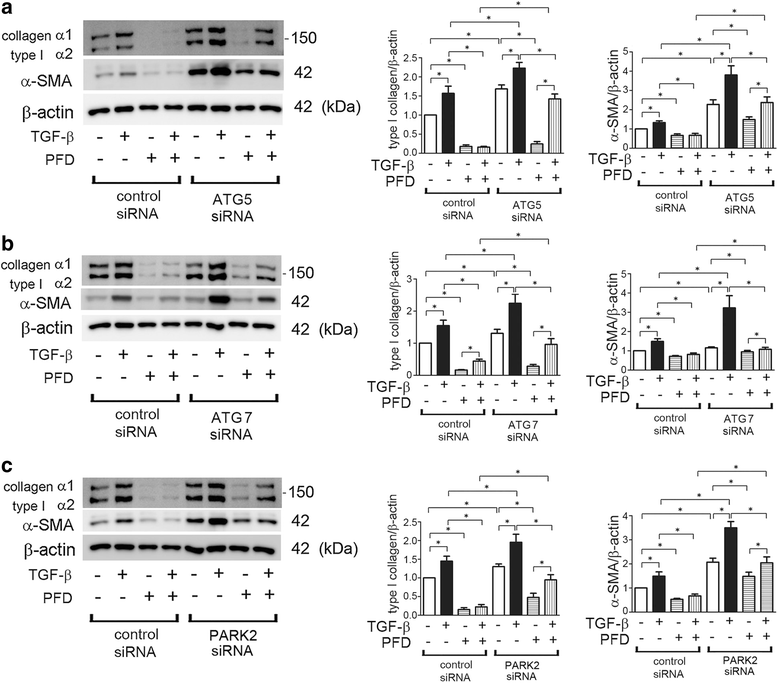
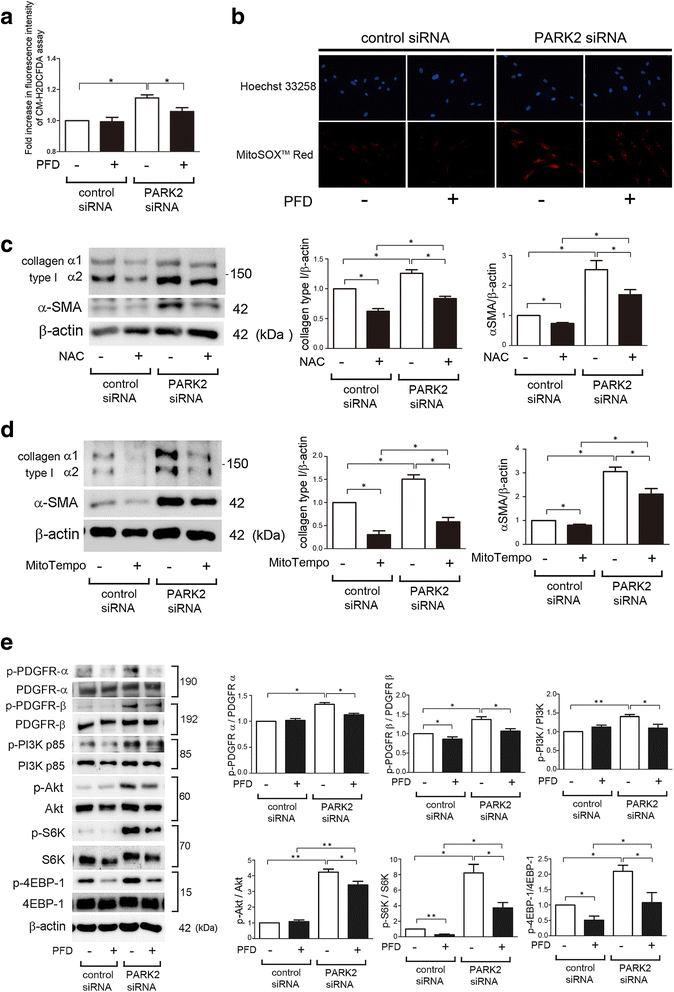
Background: Pirfenidone (PFD) is an anti-fibrotic agent used to treat idiopathic pulmonary fibrosis (IPF), but its precise mechanism of action remains elusive. Accumulation of profibrotic myofibroblasts is a crucial process for fibrotic remodeling in IPF. Recent findings show participation of autophagy/mitophagy, part of the lysosomal degradation machinery, in IPF pathogenesis. Mitophagy has been implicated in myofibroblast differentiation through regulating mitochondrial reactive oxygen species (ROS)-mediated platelet-derived growth factor receptor (PDGFR) activation. In this study, the effect of PFD on autophagy/mitophagy activation in lung fibroblasts (LF) was evaluated, specifically the anti-fibrotic property of PFD for modulation of myofibroblast differentiation during insufficient mitophagy.
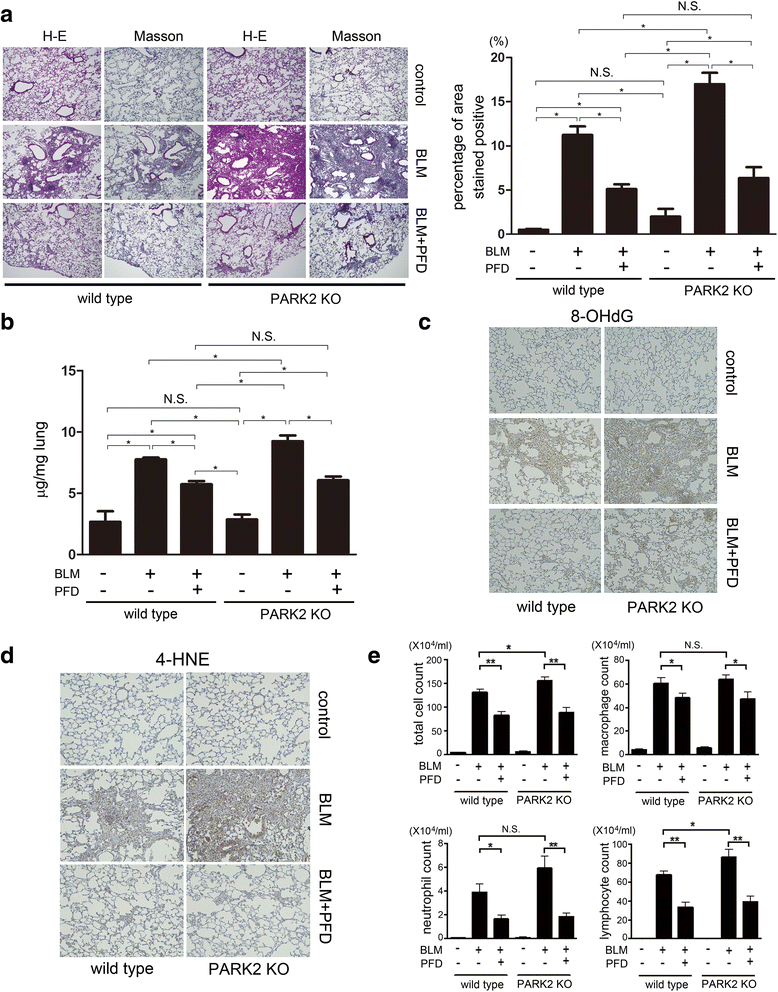
Methods: Transforming growth factor-β (TGF-β)-induced or ATG5, ATG7, and PARK2 knockdown-mediated myofibroblast differentiation in LF were used for in vitro models. The anti-fibrotic role of PFD was examined in a bleomycin (BLM)-induced lung fibrosis model using PARK2 knockout (KO) mice.
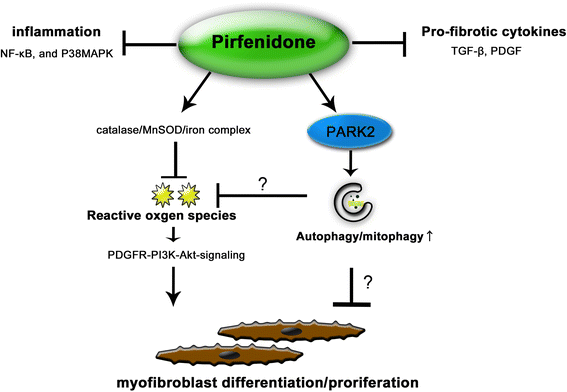
Results: We found that PFD induced autophagy/mitophagy activation via enhanced PARK2 expression, which was partly involved in the inhibition of myofibroblast differentiation in the presence of TGF-β. PFD inhibited the myofibroblast differentiation induced by PARK2 knockdown by reducing mitochondrial ROS and PDGFR-PI3K-Akt activation. BLM-treated PARK2 KO mice demonstrated augmentation of lung fibrosis and oxidative modifications compared to those of BLM-treated wild type mice, which were efficiently attenuated by PFD.
Conclusions: These results suggest that PFD induces PARK2-mediated mitophagy and also inhibits lung fibrosis development in the setting of insufficient mitophagy, which may at least partly explain the anti-fibrotic mechanisms of PFD for IPF treatment.
Keywords: Autophagy; IPF; Mitophagy; Myofibroblast; Pirfenidone.
Figures
Similar articles
-
Involvement of PARK2-Mediated Mitophagy in Idiopathic Pulmonary Fibrosis Pathogenesis.J Immunol. 2016 Jul 15;197(2):504-16. doi: 10.4049/jimmunol.1600265. Epub 2016 Jun 8. J Immunol. 2016. PMID: 27279371
-
Metformin attenuates lung fibrosis development via NOX4 suppression.Respir Res. 2016 Aug 30;17(1):107. doi: 10.1186/s12931-016-0420-x. Respir Res. 2016. PMID: 27576730 Free PMC article.
-
Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4.Autophagy. 2017 Aug 3;13(8):1420-1434. doi: 10.1080/15548627.2017.1328348. Epub 2017 Jun 14. Autophagy. 2017. PMID: 28613983 Free PMC article.
-
PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses.Inflamm Regen. 2018 Oct 24;38:18. doi: 10.1186/s41232-018-0077-6. eCollection 2018. Inflamm Regen. 2018. PMID: 30386443 Free PMC article. Review.
-
Sphingolipids in pulmonary fibrosis.Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
Cited by
-
The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases.Cells. 2021 Apr 14;10(4):897. doi: 10.3390/cells10040897. Cells. 2021. PMID: 33919784 Free PMC article. Review.
-
Alamandine/MrgD axis prevents TGF-β1-mediated fibroblast activation via regulation of aerobic glycolysis and mitophagy.J Transl Med. 2023 Jan 13;21(1):24. doi: 10.1186/s12967-022-03837-2. J Transl Med. 2023. PMID: 36635651 Free PMC article.
-
Caveolin-1 scaffolding domain peptide abrogates autophagy dysregulation in pulmonary fibrosis.Sci Rep. 2022 Jun 30;12(1):11086. doi: 10.1038/s41598-022-14832-4. Sci Rep. 2022. PMID: 35773303 Free PMC article.
-
Understanding the molecular regulatory mechanisms of autophagy in lung disease pathogenesis.Front Immunol. 2024 Oct 31;15:1460023. doi: 10.3389/fimmu.2024.1460023. eCollection 2024. Front Immunol. 2024. PMID: 39544928 Free PMC article. Review.
-
The effective-compound compatibility of JHF inhibits fibroblast activation in pulmonary fibrosis by enhancing PINK1/PARK2-mediated mitophagy.Sci Rep. 2025 Apr 8;15(1):11935. doi: 10.1038/s41598-025-95175-8. Sci Rep. 2025. PMID: 40200051 Free PMC article.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

