Sox2 regulates Müller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a
- PMID: 28577895
- PMCID: PMC5554723
- DOI: 10.1016/j.exer.2017.05.012
Sox2 regulates Müller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a
Abstract
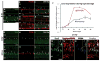
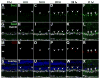
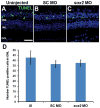
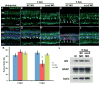
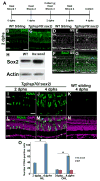
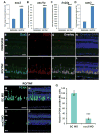
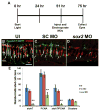
Sox2 is a well-established neuronal stem cell-associated transcription factor that regulates neural development and adult neurogenesis in vertebrates, and is one of the critical genes used to reprogram differentiated cells into induced pluripotent stem cells. We examined if Sox2 was involved in the early reprogramming-like events that Müller glia undergo as they upregulate many pluripotency- and neural stem cell-associated genes required for proliferation in light-damaged adult zebrafish retinas. In the undamaged adult zebrafish retina, Sox2 is expressed in Müller glia and a subset of amacrine cells, similar to other vertebrates. Following 31 h of light damage, Sox2 expression significantly increased in proliferating Müller glia. Morpholino-mediated knockdown of Sox2 expression resulted in decreased numbers of proliferating Müller glia, while induced overexpression of Sox2 stimulated Müller glia proliferation in the absence of retinal damage. Thus, Sox2 is necessary and sufficient for Müller glia proliferation. We investigated the role of Wnt/β-catenin signaling, which is a known regulator of sox2 expression during vertebrate retinal development. While β-catenin 2, but not β-catenin 1, was necessary for Müller glia proliferation, neither β-catenin paralog was required for sox2 expression following retinal damage. Sox2 expression was also necessary for ascl1a (neurogenic) and lin28a (reprogramming) expression, but not stat3 expression following retinal damage. Furthermore, Sox2 was required for Müller glial-derived neuronal progenitor cell amplification and expression of the pro-neural marker Tg(atoh7:EGFP). Finally, loss of Sox2 expression prevented complete regeneration of cone photoreceptors. This study is the first to identify a functional role for Sox2 during Müller glial-based regeneration of the vertebrate retina.
Keywords: Müller glia; Neuronal progenitor cell; Regeneration; Sox2; β-catenin.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




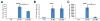


References
-
- Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES. Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–1309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

