The role of adhesion junctions in the biomechanical behaviour and osteogenic differentiation of 3D mesenchymal stem cell spheroids
- PMID: 28577903
- PMCID: PMC6392184
- DOI: 10.1016/j.jbiomech.2017.05.014
The role of adhesion junctions in the biomechanical behaviour and osteogenic differentiation of 3D mesenchymal stem cell spheroids
Abstract
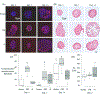
Osteogenesis of mesenchymal stem cells (MSC) can be regulated by the mechanical environment. MSCs grown in 3D spheroids (mesenspheres) have preserved multi-lineage potential, improved differentiation efficiency, and exhibit enhanced osteogenic gene expression and matrix composition in comparison to MSCs grown in 2D culture. Within 3D mesenspheres, mechanical cues are primarily in the form of cell-cell contraction, mediated by adhesion junctions, and as such adhesion junctions are likely to play an important role in the osteogenic differentiation of mesenspheres. However the precise role of N- and OB-cadherin on the biomechanical behaviour of mesenspheres remains unknown. Here we have mechanically tested mesenspheres cultured in suspension using parallel plate compression to assess the influence of N-cadherin and OB-cadherin adhesion junctions on the viscoelastic properties of the mesenspheres during osteogenesis. Our results demonstrate that N-cadherin and OB-cadherin have different effects on mesensphere viscoelastic behaviour and osteogenesis. When OB-cadherin was silenced, the viscosity, initial and long term Young's moduli and actin stress fibre formation of the mesenspheres increased in comparison to N-cadherin silenced mesenspheres and mesenspheres treated with a scrambled siRNA (Scram) at day 2. Additionally, the increased viscoelastic material properties correlate with evidence of calcification at an earlier time point (day 7) of OB-cadherin silenced mesenspheres but not Scram. Interestingly, both N-cadherin and OB-cadherin silenced mesenspheres had higher BSP2 expression than Scram at day 14. Taken together, these results indicate that N-cadherin and OB-cadherin both influence mesensphere biomechanics and osteogenesis, but play different roles.
Keywords: Biomechanics; Cadherin; Mesenchymal stem cell; Suspension culture; Viscoelastic.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
All authors declare no financial or competing interests.
Figures




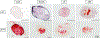

References
-
- Cook MM, Futrega K, Osiecki M, Kabiri M, Kul B, Rice A, Atkinson K, Brooke G, Doran M, 2012. Micromarrows—three-dimensional coculture of hematopoietic stem cells and mesenchymal stromal cells. Tissue Eng. Part C: Methods 18 (5), 319–328. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

