SOX7 expression is critically required in FLK1-expressing cells for vasculogenesis and angiogenesis during mouse embryonic development
- PMID: 28577909
- PMCID: PMC5496588
- DOI: 10.1016/j.mod.2017.05.004
SOX7 expression is critically required in FLK1-expressing cells for vasculogenesis and angiogenesis during mouse embryonic development
Abstract
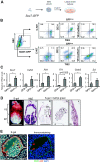
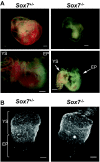
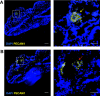
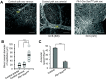
The transcriptional program that regulates the differentiation of endothelial precursor cells into a highly organized vascular network is still poorly understood. Here we explore the role of SOX7 during this process, performing a detailed analysis of the vascular defects resulting from either a complete deficiency in Sox7 expression or from the conditional deletion of Sox7 in FLK1-expressing cells. We analysed the consequence of Sox7 deficiency from E7.5 onward to determine from which stage of development the effect of Sox7 deficiency can be observed. We show that while Sox7 is expressed at the onset of endothelial specification from mesoderm, Sox7 deficiency does not impact the emergence of the first endothelial progenitors. However, by E8.5, clear signs of defective vascular development are already observed with the presence of highly unorganised endothelial cords rather than distinct paired dorsal aorta. By E10.5, both Sox7 complete knockout and FLK1-specific deletion of Sox7 lead to widespread vascular defects. In contrast, while SOX7 is expressed in the earliest specified blood progenitors, the VAV-specific deletion of Sox7 does not affect the hematopoietic system. Together, our data reveal the unique role of SOX7 in vasculogenesis and angiogenesis during embryonic development.
Keywords: Endothelium; SOXF; Vascular development.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures






References
-
- de Boer J., Williams A., Skavdis G., Harker N., Coles M., Tolaini M., Norton T., Williams K., Roderick K., Potocnik A.J., Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003;33:314–325. - PubMed
-
- Chung M.I., Ma A.C., Fung T.K., Leung A.Y. Characterization of Sry-related HMG box group F genes in zebrafish hematopoiesis. Exp. Hematol. 2011;39(986–998) - PubMed
-
- Drake C.J., Fleming P.A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

