A proof-of-concept study for developing integrated two-photon microscopic and magnetic resonance imaging modality at ultrahigh field of 16.4 tesla
- PMID: 28578390
- PMCID: PMC5457450
- DOI: 10.1038/s41598-017-02864-0
A proof-of-concept study for developing integrated two-photon microscopic and magnetic resonance imaging modality at ultrahigh field of 16.4 tesla
Abstract
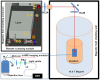
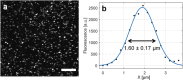
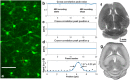
Functional magnetic resonance imaging (fMRI) based on the blood oxygen level dependent (BOLD) contrast has gained a prominent position in neuroscience for imaging neuronal activity and studying effective brain connectivity under working state and functional connectivity at resting state. However, the fundamental questions in regards to fMRI technology: how the BOLD signal inferences the underlying microscopic neuronal activity and physiological changes and what is the ultimate specificity of fMRI for functional mapping of microcircuits, remain unanswered. The capability of simultaneous fMRI measurement and functional microscopic imaging in a live brain thus holds the key to link the microscopic and mesoscopic neural dynamics to the macroscopic brain activity at the central nervous system level. Here we report the first demonstration to integrate high-resolution two-photon fluorescence microscopy (TPM) with a 16.4 tesla MRI system, which proves the concept and feasibility for performing simultaneous high-resolution fMRI and TPM imaging at ultrahigh magnetic field.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

