Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model
- PMID: 28578396
- PMCID: PMC5457429
- DOI: 10.1038/s41598-017-02806-w
Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model
Abstract
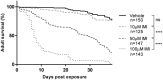
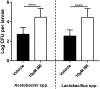
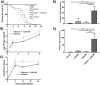
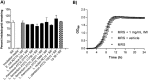
Pesticides are used extensively in food production to maximize crop yields. However, neonicotinoid insecticides exert unintentional toxicity to honey bees (Apis mellifera) that may partially be associated with massive population declines referred to as colony collapse disorder. We hypothesized that imidacloprid (common neonicotinoid; IMI) exposure would make Drosophila melanogaster (an insect model for the honey bee) more susceptible to bacterial pathogens, heat stress, and intestinal dysbiosis. Our results suggested that the immune deficiency (Imd) pathway is necessary for D. melanogaster survival in response to IMI toxicity. IMI exposure induced alterations in the host-microbiota as noted by increased indigenous Acetobacter and Lactobacillus spp. Furthermore, sub-lethal exposure to IMI resulted in decreased D. melanogaster survival when simultaneously exposed to bacterial infection and heat stress (37 °C). This coincided with exacerbated increases in TotA and Dpt (Imd downstream pro-survival and antimicrobial genes, respectively) expression compared to controls. Supplementation of IMI-exposed D. melanogaster with Lactobacillus plantarum ATCC 14917 mitigated survival deficits following Serratia marcescens (bacterial pathogen) septic infection. These findings support the insidious toxicity of neonicotinoid pesticides and potential for probiotic lactobacilli to reduce IMI-induced susceptibility to infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Lu YH, et al. Species composition and seasonal abundance of pestiferous plant bugs (Hemiptera: Miridae) on Bt Cotton in China. Crop Prot. 2008;27:465–472. doi: 10.1016/j.cropro.2007.07.017. - DOI
-
- Greene JK, Turnipseed SG, Sullivan MJ, Herzog GA. Boll damage by southern green stink bug (Hemiptera: Pentatomidae) and tarnished plant bug (Hemiptera: Miridae) caged on transgenic Bacillus thuringiensis cotton. J. Econ. Entomol. 1999;92:941–944. doi: 10.1093/jee/92.4.941. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

