Progressive resistance training in head and neck cancer patients during concomitant chemoradiotherapy -- design of the DAHANCA 31 randomized trial
- PMID: 28578654
- PMCID: PMC5457597
- DOI: 10.1186/s12885-017-3388-0
Progressive resistance training in head and neck cancer patients during concomitant chemoradiotherapy -- design of the DAHANCA 31 randomized trial
Abstract
Background: Head and neck cancer patients undergoing concomitant chemoradiotherapy (CCRT) frequently experience loss of muscle mass and reduced functional performance. Positive effects of exercise training are reported for many cancer types but biological mechanisms need further elucidation. This randomized study investigates whether progressive resistance training (PRT) may attenuate loss of muscle mass and functional performance. Furthermore, biochemical markers and muscle biopsies will be investigated trying to link biological mechanisms to training effects.
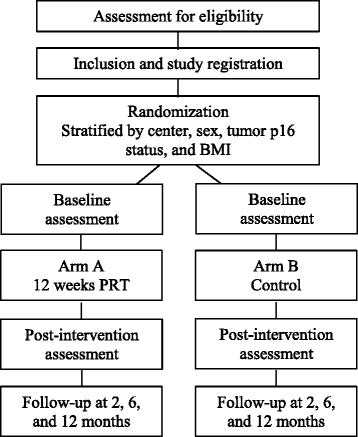
Methods: At the Departments of Oncology at Herlev and Aarhus University Hospitals, patients with stage III/IV squamous cell carcinoma of the head and neck, scheduled for CCRT are randomized 1:1 to either a 12-week PRT program or control group, both with 1 year follow-up. Planned enrollment is 72 patients, and stratification variables are study site, sex, p16-status, and body mass index. Primary endpoint is difference in change in lean body mass (LBM) after 12 weeks of PRT, assessed by dual-energy X-ray absorptiometry (DXA). The hypothesis is that 12 weeks of PRT can attenuate the loss of LBM by at least 25%. Secondary endpoints include training adherence, changes in body composition, muscle strength, functional performance, weight, adverse events, dietary intake, self-reported physical activity, quality of life, labor market affiliation, blood biochemistry, plasma cytokine concentrations, NK-cell frequency in blood, sarcomeric protein content in muscles, as well as muscle fiber type and fiber size in muscle biopsies. Muscle biopsies are optional.
Discussion: This randomized study investigates the impact of a 12-week progressive resistance training program on lean body mass and several other physiological endpoints, as well as impact on adverse events and quality of life. Furthermore, a translational approach is integrated with extensive biological sampling and exploration into cytokines and mechanisms involved. The current paper discusses decisions and methods behind exercise in head and neck cancer patients undergoing concomitant chemoradiotherapy.
Trial registration: Approved by the Regional Ethics Committee for the Capital Region of Denmark (protocol id: H-15003725) and registered retrospectively at ClinicalTrials.gov ( NCT02557529 ) September 11th 2015.
Keywords: Body composition; Body weight; Chemoradiotherapy; Exercise; Head and neck cancer; Head and neck squamous cell carcinoma; Lean body mass; Physical activity; Progressive resistance training; Weight.
Similar articles
-
Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy--results from the randomized DAHANCA 25B trial.Radiother Oncol. 2013 Aug;108(2):314-9. doi: 10.1016/j.radonc.2013.07.002. Epub 2013 Aug 6. Radiother Oncol. 2013. PMID: 23932192 Clinical Trial.
-
Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients--the DAHANCA 25A study.Acta Oncol. 2013 Feb;52(2):310-8. doi: 10.3109/0284186X.2012.741325. Epub 2012 Nov 28. Acta Oncol. 2013. PMID: 23190359 Clinical Trial.
-
Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry.Head Neck. 2014 Sep;36(9):1356-62. doi: 10.1002/hed.23461. Epub 2013 Nov 21. Head Neck. 2014. PMID: 23970480 Clinical Trial.
-
Body composition changes in patients with head and neck cancer under active treatment: a scoping review.Support Care Cancer. 2020 Oct;28(10):4613-4625. doi: 10.1007/s00520-020-05487-w. Epub 2020 Jun 13. Support Care Cancer. 2020. PMID: 32533436
-
Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis.J Cancer Surviv. 2017 Jun;11(3):339-349. doi: 10.1007/s11764-016-0592-x. Epub 2017 Jan 4. J Cancer Surviv. 2017. PMID: 28054255 Review.
Cited by
-
Bioelectrical impedance analysis as a quantitative measure of sarcopenia in head and neck cancer patients treated with radiotherapy.Radiother Oncol. 2021 Jun;159:21-27. doi: 10.1016/j.radonc.2021.03.005. Epub 2021 Mar 15. Radiother Oncol. 2021. PMID: 33736997 Free PMC article.
-
Sarcopenia and Treatment Toxicity in Older Adults Undergoing Chemoradiation for Head and Neck Cancer: Identifying Factors to Predict Frailty.Cancers (Basel). 2022 Apr 22;14(9):2094. doi: 10.3390/cancers14092094. Cancers (Basel). 2022. PMID: 35565223 Free PMC article.
-
Effects of Exercise Training during Concomitant Chemoradiation Therapy in Head-and-Neck Cancer Patients: A Systematic Review.Indian J Palliat Care. 2020 Oct-Dec;26(4):531-532. doi: 10.4103/IJPC.IJPC_14_20. Epub 2020 Nov 19. Indian J Palliat Care. 2020. PMID: 33623318 Free PMC article.
-
Feasibility and acceptability of home-based strength training in endometrial cancer survivors.J Cancer Surviv. 2023 Feb;17(1):120-129. doi: 10.1007/s11764-021-00990-3. Epub 2021 Mar 6. J Cancer Surviv. 2023. PMID: 33675013 Free PMC article. Clinical Trial.
-
Elucidating the mechanisms of lifestyle interventions in mitigating radiotherapy adverse effects: a scoping review.BMJ Oncol. 2025 Jul 10;4(1):e000615. doi: 10.1136/bmjonc-2024-000615. eCollection 2025. BMJ Oncol. 2025. PMID: 40662157 Free PMC article. Review.
References
-
- Jackson W, Alexander N, Schipper M, Fig L, Feng F, Jolly S. Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry. Head Neck. 2014;36(9):1356–1362. - PubMed
-
- Langius JE, Van Dijk AM, Doornaert P, Kruizenga HM, Langendijk JA, Leemans CR, et al. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr Cancer. 2013;65(1):76–83. doi: 10.1080/01635581.2013.741749. - DOI - PubMed
-
- Platek ME, Myrick E, Mccloskey SA, Gupta V, Reid ME, Wilding GE, et al. Pretreatment weight status and weight loss among head and neck cancer patients receiving definitive concurrent chemoradiation therapy: implications for nutrition integrated treatment pathways. Support Care Cancer. 2013;21(10):2825–2833. doi: 10.1007/s00520-013-1861-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical