Meal Timing Regulates the Human Circadian System
- PMID: 28578930
- PMCID: PMC5483233
- DOI: 10.1016/j.cub.2017.04.059
Meal Timing Regulates the Human Circadian System
Abstract
Circadian rhythms, metabolism, and nutrition are intimately linked [1, 2], although effects of meal timing on the human circadian system are poorly understood. We investigated the effect of a 5-hr delay in meals on markers of the human master clock and multiple peripheral circadian rhythms. Ten healthy young men undertook a 13-day laboratory protocol. Three meals (breakfast, lunch, dinner) were given at 5-hr intervals, beginning either 0.5 (early) or 5.5 (late) hr after wake. Participants were acclimated to early meals and then switched to late meals for 6 days. After each meal schedule, participants' circadian rhythms were measured in a 37-hr constant routine that removes sleep and environmental rhythms while replacing meals with hourly isocaloric snacks. Meal timing did not alter actigraphic sleep parameters before circadian rhythm measurement. In constant routines, meal timing did not affect rhythms of subjective hunger and sleepiness, master clock markers (plasma melatonin and cortisol), plasma triglycerides, or clock gene expression in whole blood. Following late meals, however, plasma glucose rhythms were delayed by 5.69 ± 1.29 hr (p < 0.001), and average glucose concentration decreased by 0.27 ± 0.05 mM (p < 0.001). In adipose tissue, PER2 mRNA rhythms were delayed by 0.97 ± 0.29 hr (p < 0.01), indicating that human molecular clocks may be regulated by feeding time and could underpin plasma glucose changes. Timed meals therefore play a role in synchronizing peripheral circadian rhythms in humans and may have particular relevance for patients with circadian rhythm disorders, shift workers, and transmeridian travelers.
Keywords: actigraphy; chrononutrition; clock gene; food timing; glucose homeostasis; jet lag; meal timing; peripheral clocks; shift work; white adipose tissue.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
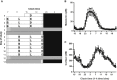
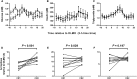

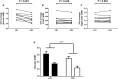
Comment in
-
Circadian Biology: Uncoupling Human Body Clocks by Food Timing.Curr Biol. 2017 Jul 10;27(13):R656-R658. doi: 10.1016/j.cub.2017.05.057. Curr Biol. 2017. PMID: 28697366
References
-
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. - PubMed
-
- Schibler U., Gotic I., Saini C., Gos P., Curie T., Emmenegger Y., Sinturel F., Gosselin P., Gerber A., Fleury-Olela F. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant. Biol. 2015;80:223–232. - PubMed
-
- Roenneberg T., Merrow M. The circadian clock and human health. Curr. Biol. 2016;26:R432–R443. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

