Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy
- PMID: 28578991
- PMCID: PMC5542805
- DOI: 10.1016/j.ymthe.2017.05.006
Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy
Abstract
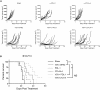
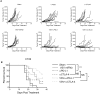
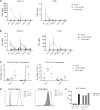
Vesicular stomatitis virus encoding the IFNβ transgene (VSV-IFNβ) is a mediator of potent oncolytic activity and is undergoing clinical evaluation for the treatment of solid tumors. Emerging preclinical and clinical data suggest treatment of tumors with oncolytic viruses may sensitize tumors to checkpoint inhibitors and increase the anti-tumor immune response. New generations of immuno-oncology molecules including T cell agonists are entering clinical development and could be hypothesized to enhance the activity of oncolytic viruses, including VSV-IFNβ. Here, we show that VSV-IFNβ exhibits multiple mechanisms of action, including direct cell killing, stimulation of an innate immune response, recruitment of CD8 T cells, and depletion of T regulatory cells. Moreover, VSV-IFNβ promotes the establishment of a CD8 T cell response to endogenous tumor antigens. Our data demonstrate a significant enhancement of anti-tumor function for VSV-IFNβ when combined with checkpoint inhibitors, but not OX40 agonists. While the addition of checkpoint inhibitors to VSV-IFNβ generated robust tumor growth inhibition, it resulted in no increase in viral replication, transgene expression, or immunophenotypic changes beyond treatment with VSV-IFNβ alone. We hypothesize that tumor-specific T cells generated by VSV-IFNβ retain activity due to a lack of immune exhaustion when checkpoint inhibitors were used.
Keywords: VSV; agonist; checkpoint inhibitor; immune-oncology; oncolytic virus.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Chiocca E.A. Oncolytic viruses. Nat. Rev. Cancer. 2002;2:938–950. - PubMed
-
- Liu T.-C., Galanis E., Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. - PubMed
-
- Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

