Meat Intake and the Dose of Vitamin B3 - Nicotinamide: Cause of the Causes of Disease Transitions, Health Divides, and Health Futures?
- PMID: 28579801
- PMCID: PMC5419340
- DOI: 10.1177/1178646917704662
Meat Intake and the Dose of Vitamin B3 - Nicotinamide: Cause of the Causes of Disease Transitions, Health Divides, and Health Futures?
Abstract
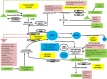
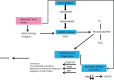
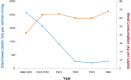
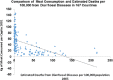
Meat and vitamin B3 - nicotinamide - intake was high during hunter-gatherer times. Intake then fell and variances increased during and after the Neolithic agricultural revolution. Health, height, and IQ deteriorated. Low dietary doses are buffered by 'welcoming' gut symbionts and tuberculosis that can supply nicotinamide, but this co-evolved homeostatic metagenomic strategy risks dysbioses and impaired resistance to pathogens. Vitamin B3 deficiency may now be common among the poor billions on a low-meat diet. Disease transitions to non-communicable inflammatory disorders (but longer lives) may be driven by positive 'meat transitions'. High doses of nicotinamide lead to reduced regulatory T cells and immune intolerance. Loss of no longer needed symbiotic 'old friends' compounds immunological over-reactivity to cause allergic and auto-immune diseases. Inhibition of nicotinamide adenine dinucleotide consumers and loss of methyl groups or production of toxins may cause cancers, metabolic toxicity, or neurodegeneration. An optimal dosage of vitamin B3 could lead to better health, but such a preventive approach needs more equitable meat distribution. Some people may require personalised doses depending on genetic make-up or, temporarily, when under stress.
Keywords: Diet; Parkinson; allergies; cancer; disease transitions; environmental enteropathy; health inequality; hygiene hypothesis; hyper-vitaminosis B3; metabolic syndrome; nicotinamide; pellagra; tryptophan.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures












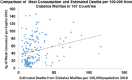
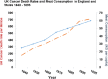












References
-
- Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28:S2–S9. - PubMed
-
- Omran AR. The epidemiologic transition theory. A preliminary update. J Trop Pediatr. 1983;29:305–316. - PubMed
-
- Lindeberg S. Food and Western Disease: Health and Nutrition from an Evolutionary Perspective. Hoboken, NJ: Wiley; 2009.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

