The regulation of steroid receptors by epigallocatechin-3-gallate in breast cancer cells
- PMID: 28579831
- PMCID: PMC5447698
- DOI: 10.2147/BCTT.S131334
The regulation of steroid receptors by epigallocatechin-3-gallate in breast cancer cells
Abstract
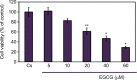
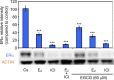
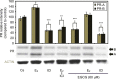
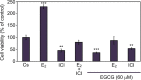
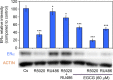
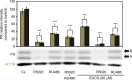
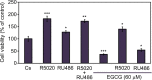
It has been reported that phytoestrogen epigallocatechin gallate (EGCG) suppresses cancer cell proliferation and may have antitumor properties. In this study, we analyzed the effects of EGCG on estrogen receptor α (ERα) and progesterone receptor in hormone-dependent T-47D breast cancer cells. Western blot analysis revealed EGCG induced a concentration-dependent decrease in ERα protein levels, with a 56% reduction occurring with 60 µM EGCG when compared to controls. Downregulation of ERα protein levels was observed after 24-hour co-treatment of T-47D cells with 60 µM EGCG and 10 nM 17β-estradiol (E2). The proliferative effect of E2 on cell viability was reversed when treated in combination with EGCG. In contrast, the combination of EGCG with the pure ER antagonist, ICI 182, 780, showed no further reduction in cell number as only 5% of the cells were viable after 6 days of treatment. These studies may provide further understanding of the interactions among flavonoids and steroid receptors in breast cancer cells.
Keywords: ER; PR; T-47D; antiestrogens; phytoestrogen.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Wiechmann L, Kuerer HM. The molecular journey from ductal carcinoma in situ to invasive breast cancer. Cancer. 2008;112(10):2130–2142. - PubMed
-
- Siebert AE, Sanchez AL, Dinda S, Moudgil VK. Effects of estrogen metabolite 2-methoxyestradiol on tumor suppressor protein p53 and proliferation of breast cancer cells. Syst Biol Reprod Med. 2011;57(6):279–287. - PubMed
-
- De Angelis M, Stossi F, Waibel M, Katzenellenbogen BS, Katzenellenbogen JA. Isocoumarins as estrogen receptor beta selective ligands: isomers of isoflavone phytoestrogens and their metabolites. Bioorg Med Chem. 2005;13(23):6529–6542. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

