Hypoglycemia: a review of definitions used in clinical trials evaluating antihyperglycemic drugs for diabetes
- PMID: 28579834
- PMCID: PMC5448700
- DOI: 10.2147/CLEP.S129268
Hypoglycemia: a review of definitions used in clinical trials evaluating antihyperglycemic drugs for diabetes
Abstract
Objective: To understand the severity and potential impact of heterogeneity in definitions of hypoglycemia used in diabetes research, we aimed to review the hypoglycemia definitions adopted in randomized controlled trials (RCTs).
Methods: We reviewed 109 RCTs included in the Canadian Agency for Drugs and Technologies in Health reports for the second- and third-line therapy for the patients with type 2 diabetes (T2D).
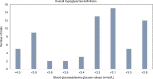
Results: Nearly 60% (n=66) of the studies reviewed presented the definitions for overall hypoglycemia, and another 20% (n=22) of the studies reported the results for hypoglycemia but did not report a definition. Among these 66 studies, only 9 (14%) followed the American Diabetes Association/European Medicines Agency specified guidelines to define hypoglycemia, with an exact threshold of plasma glucose ≤3.9 mmol/L. Fifty-two of the 66 studies (79%) used a threshold considerably lower than the recommended ≤3.9 mmol/L, and 16 studies used a threshold between 3.8 and 4.0 mmol/L. The proportion of the trials that used a cutoff value of <3.1 mmol/L appeared to be slightly similar among the more commonly used non-insulin treatments, GLP-1s (7 of 18 [39%]), thiazolidinediones (TZDs; 6 of 11 [55%]), DPP-4s (12 of 19 [64%]), and sulfonylureas (11 of 20 [55%]). Among trials with intermediate-long-acting insulins (neutral protamine Hagedorn insulin, detemir, glargine), 7 of 26 trials (27%) used a cutoff of <3.1 mmol/L. The definition of severe hypoglycemia was also subject to substantial heterogeneity, in both the utilized threshold and accompanying soft definitions.
Conclusion: This review demonstrates that substantial heterogeneity exists in the definition of overall, severe/major, and nocturnal hypoglycemia across RCTs investigating T2D interventions.
Keywords: diabetes; heterogeneity; hypoglycemia; randomized controlled trials; review.
Conflict of interest statement
Disclosure Funding for this study was granted by a Mitacs Elevate postdoctoral fellowship provided to CB. This fellowship allowed for the collaboration between researchers at Simon Fraser University and Precision Health Economics. ED, GS, KT, and EJM were employees of Precision Health Economics at the time of manuscript submission. MJ is a professor at Simon Fraser University. The authors report no other conflicts of interest in this work.
Figures
References
-
- International Diabetes Federation IDF Diabetes Atlas. 6th ed. 2013. [Accessed December 5, 2016]. Available from: https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.
-
- Group UKHS. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–1147. - PubMed
-
- Canadian Diabetes Association Clinical Practice Guidelines Expert C. Clayton D, Woo V, Yale JF. Hypoglycemia. Can J Diabetes. 2013;37(suppl 1):S69–S71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources