The Crosstalk between the Gut Microbiota and Mitochondria during Exercise
- PMID: 28579962
- PMCID: PMC5437217
- DOI: 10.3389/fphys.2017.00319
The Crosstalk between the Gut Microbiota and Mitochondria during Exercise
Abstract
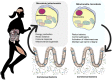
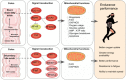
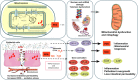
Many physiological changes occur in response to endurance exercise in order to adapt to the increasing energy needs, mitochondria biogenesis, increased reactive oxygen species (ROS) production and acute inflammatory responses. Mitochondria are organelles within each cell that are crucial for ATP production and are also a major producer of ROS and reactive nitrogen species during intense exercise. Recent evidence shows there is a bidirectional interaction between mitochondria and microbiota. The gut microbiota have been shown to regulate key transcriptional co-activators, transcription factors and enzymes involved in mitochondrial biogenesis such as PGC-1α, SIRT1, and AMPK genes. Furthermore, the gut microbiota and its metabolites, such as short chain fatty acids and secondary bile acids, also contribute to host energy production, ROS modulation and inflammation in the gut by attenuating TNFα- mediated immune responses and inflammasomes such as NLRP3. On the other hand, mitochondria, particularly mitochondrial ROS production, have a crucial role in regulating the gut microbiota via modulating intestinal barrier function and mucosal immune responses. Recently, it has also been shown that genetic variants within the mitochondrial genome, could affect mitochondrial function and therefore the intestinal microbiota composition and activity. Diet is also known to dramatically modulate the composition of the gut microbiota. Therefore, studies targeting the gut microbiota can be useful for managing mitochondrial related ROS production, pro-inflammatory signals and metabolic limits in endurance athletes.
Keywords: endurance; energy; gut microbiota; inflammation; mitochondria; oxidative stress.
Figures
References
-
- Andersson S. G. E., Zomorodipour A., Andersson J. O., Sicheritz-Pontén T., Alsmark U. C. M., Podowski R. M., et al. . (1998). The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–140. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

