Electronic properties of the boroxine-gold interface: evidence of ultra-fast charge delocalization
- PMID: 28580111
- PMCID: PMC5436552
- DOI: 10.1039/c6sc05632f
Electronic properties of the boroxine-gold interface: evidence of ultra-fast charge delocalization
Abstract
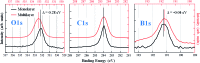
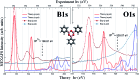
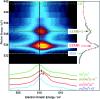
We performed a combined experimental and theoretical study of the assembly of phenylboronic acid on the Au(111) surface, which is found to lead to the formation of triphenylboroxines by spontaneous condensation of trimers of molecules. The interface between the boroxine group and the gold surface has been characterized in terms of its electronic properties, revealing the existence of an ultra-fast charge delocalization channel in the proximity of the oxygen atoms of the heterocyclic group. More specifically, the DFT calculations show the presence of an unoccupied electronic state localized on both the oxygen atoms of the adsorbed triphenylboroxine and the gold atoms of the topmost layer. By means of resonant Auger electron spectroscopy, we demonstrate that this interface state represents an ultra-fast charge delocalization channel. Boroxine groups are among the most widely adopted building blocks in the synthesis of covalent organic frameworks on surfaces. Our findings indicate that such systems, typically employed as templates for the growth of organic films, can also act as active interlayers that provide an efficient electronic transport channel bridging the inorganic substrate and organic overlayer.
Figures


References
-
- Hall D. G., Boronic Acids. Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, 2006.
-
- Nishiyabu R., Kubo Y., James T. D., Fossey J. S. Chem. Commun. 2011;47:1124–1150. - PubMed
-
- Kubo Y., Nishiyabu R., James T. D. Chem. Commun. 2014;51:2005–2020. - PubMed
-
- Feng X., Ding X., Jiang D. Chem. Soc. Rev. 2012;41:6010. - PubMed
-
- Waller P. J., Gándara F., Yaghi O. M. Acc. Chem. Res. 2015;48:3053–3063. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

