A 2-aza-Cope reactivity-based platform for ratiometric fluorescence imaging of formaldehyde in living cells
- PMID: 28580121
- PMCID: PMC5434806
- DOI: 10.1039/c7sc00748e
A 2-aza-Cope reactivity-based platform for ratiometric fluorescence imaging of formaldehyde in living cells
Abstract
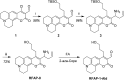
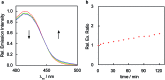
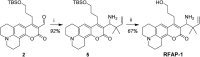
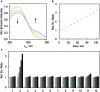
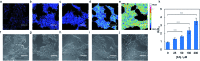
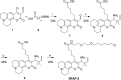
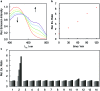
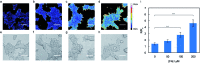
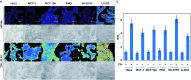
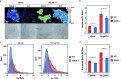
Formaldehyde (FA) is a major reactive carbonyl species (RCS) that is naturally produced in living systems through a diverse array of cellular pathways that span from epigenetic regulation to the metabolic processing of endogenous metabolites. At the same time, however, aberrant elevations in FA levels contribute to pathologies ranging from cancer and diabetes to heart, liver, and neurodegenerative diseases. Disentangling the complex interplay between FA physiology and pathology motivates the development of chemical tools that can enable the selective detection of this RCS in biological environments with spatial and temporal fidelity. We report the design, synthesis, and biological evaluation of ratiometric formaldehyde probe (RFAP) indicators for the excitation-ratiometric fluorescence imaging of formaldehyde production in living systems. RFAP-1 and RFAP-2 utilize FA-dependent aza-Cope reactivity to convert an alkylamine-functionalized coumarin platform into its aldehyde congener with a ca. 50 nm shift in the excitation wavelength. The probes exhibit visible excitation and emission profiles, and high selectivity for FA over a variety of RCS and related reactive biological analytes, including acetaldehyde, with up to a 6-fold change in the fluorescence ratio. The RFAP indicators can be used to monitor changes in FA levels in biological samples by live-cell imaging and/or flow cytometry. Moreover, RFAP-2 is capable of visualizing differences in the resting FA levels between wild-type cells and models with a gene knockout of ADH5, a major FA-metabolizing enzyme, establishing the utility of this ratiometric detection platform for identifying and probing sources of FA fluxes in biology.
Figures


References
-
- Liteplo R. G., Beauchamp R., Meek M. E. and Chénier R., in Formaldehyde (Concise International Chemical Assessment Documents), World Health Organization, Geneva, 2002.
-
- Baan R., Grosse Y., Straif K., Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V. Lancet Oncol. 2009;10:1143–1144. - PubMed
-
- Miao J. and He R., Chronic Formaldehyde-Mediated Impairments and Age-Related Dementia, InTech, Shanghai, 2012.
-
- Tulpule K., Dringen R. J. Neurochem. 2013;127:7–21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

