Cerebral Microvascular Accumulation of Tau Oligomers in Alzheimer's Disease and Related Tauopathies
- PMID: 28580182
- PMCID: PMC5440106
- DOI: 10.14336/AD.2017.0112
Cerebral Microvascular Accumulation of Tau Oligomers in Alzheimer's Disease and Related Tauopathies
Abstract
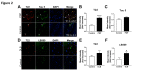
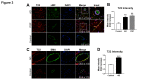
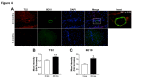
The importance of vascular contributions to cognitive impairment and dementia (VCID) associated with Alzheimer's disease (AD) and related neurodegenerative diseases is increasingly recognized, however, the underlying mechanisms remain obscure. There is growing evidence that in addition to Aβ deposition, accumulation of hyperphosphorylated oligomeric tau contributes significantly to AD etiology. Tau oligomers are toxic and it has been suggested that they propagate in a "prion-like" fashion, inducing endogenous tau misfolding in cells. Their role in VCID, however, is not yet understood. The present study was designed to determine the severity of vascular deposition of oligomeric tau in the brain in patients with AD and related tauopathies, including dementia with Lewy bodies (DLB) and progressive supranuclear palsy (PSP). Further, we examined a potential link between vascular deposition of fibrillar Aβ and that of tau oligomers in the Tg2576 mouse model. We found that tau oligomers accumulate in cerebral microvasculature of human patients with AD and PSP, in association with vascular endothelial and smooth muscle cells. Cerebrovascular deposition of tau oligomers was also found in DLB patients. We also show that tau oligomers accumulate in cerebral microvasculature of Tg2576 mice, partially in association with cerebrovascular Aβ deposits. Thus, our findings add to the growing evidence for multifaceted microvascular involvement in the pathogenesis of AD and other neurodegenerative diseases. Accumulation of tau oligomers may represent a potential novel mechanism by which functional and structural integrity of the cerebral microvessels is compromised.
Keywords: Alzheimer’s disease; brain vascular dysfunction; cerebrovascular dysfunction; cerebrovasculature; oligomers; tau; tauopathies.
Figures

References
-
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. (2005). Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol, 57: 789-794 - PubMed
-
- Cantin S, Villien M, Moreaud O, Tropres I, Keignart S, Chipon E, et al. (2011). Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. NeuroImage, 58: 579-587 - PubMed
-
- Iadecola C (2004). Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci, 5: 347-360 - PubMed
-
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. (2016). The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol, 36: 281-288 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
