Transposable elements in Drosophila
- PMID: 28580197
- PMCID: PMC5443660
- DOI: 10.1080/2159256X.2017.1318201
Transposable elements in Drosophila
Abstract
Transposable elements (TEs) are mobile genetic elements that can mobilize within host genomes. As TEs comprise more than 40% of the human genome and are linked to numerous diseases, understanding their mechanisms of mobilization and regulation is important. Drosophila melanogaster is an ideal model organism for the study of eukaryotic TEs as its genome contains a diverse array of active TEs. TEs universally impact host genome size via transposition and deletion events, but may also adopt unique functional roles in host organisms. There are 2 main classes of TEs: DNA transposons and retrotransposons. These classes are further divided into subgroups of TEs with unique structural and functional characteristics, demonstrating the significant variability among these elements. Despite this variability, D. melanogaster and other eukaryotic organisms utilize conserved mechanisms to regulate TEs. This review focuses on the transposition mechanisms and regulatory pathways of TEs, and their functional roles in D. melanogaster.
Keywords: LTR retrotransposons; P elements; TEs; TIR transposons; helitrons; non-LTR retrotransposons; retrovirus; transposons.
Figures

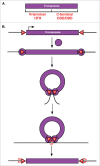
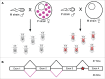
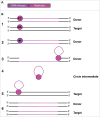
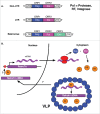
References
-
- Pagel M, Johnstone RA. Variation across species in the size of the nuclear genome supports the junk-DNA explanation for the C-value paradox. Proc Biol Sci 1992; 249:119-24; PMID:1360673; https://doi.org/ 10.1098/rspb.1992.0093 - DOI - PubMed
-
- Vieira C, Nardon C, Arpin C, Lepetit D, Biemont C. Evolution of genome size in Drosophila. Is the invader's genome being invaded by transposable elements? Mol Biol Evol 2002; 19:1154-161; PMID:12082134 - PubMed
-
- Bosco G, Campbell P, Leiva-Neto JT, Markow TA. Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 2007; 177:1277-90; PMID:18039867; https://doi.org/ 10.1534/genetics.107.075069 - DOI - PMC - PubMed
-
- Gregory TR, Johnston JS. Genome size diversity in the family Drosophilidae. Heredity (Edinb) 2008; 101:228-38; PMID:18523443; https://doi.org/ 10.1038/hdy.2008.49 - DOI - PubMed
-
- SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer PS, Edwards KJ, Lee M, Avramova Z, et al.. Nested retrotransposons in the intergenic regions of the maize genome. Science 1996; 274:765-8; PMID:8864112 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
