In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment
- PMID: 28580765
- PMCID: PMC5862030
- DOI: 10.1002/jbm.b.33936
In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment
Abstract
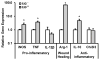
Scarring of the vocal fold lamina propria can lead to debilitating voice disorders that can significantly impair quality of life. The reduced pliability of the scar tissue-which diminishes proper vocal fold vibratory efficiency-results in part from abnormal extracellular matrix (ECM) deposition by vocal fold fibroblasts (VFF) that have taken on a fibrotic phenotype. To address this issue, bioactive materials containing cytokines and/or growth factors may provide a platform to transition fibrotic VFF within the scarred tissue toward an anti-fibrotic phenotype, thereby improving the quality of ECM within the scar tissue. However, for such an approach to be most effective, the acute host response resulting from biomaterial insertion/injection likely also needs to be considered. The goal of the present work was to evaluate the anti-fibrotic and anti-inflammatory capacity of an injectable hydrogel containing tethered basic fibroblast growth factor (bFGF) in the dual context of scar and biomaterial-induced acute inflammation. An in vitro co-culture system was utilized containing both activated, fibrotic VFF and activated, pro-inflammatory macrophages (MΦ) within a 3D poly(ethylene glycol) diacrylate (PEGDA) hydrogel containing tethered bFGF. Following 72 h of culture, alterations in VFF and macrophage phenotype were evaluated relative to mono-culture and co-culture controls. In our co-culture system, bFGF reduced the production of fibrotic markers collagen type I, α smooth muscle actin, and biglycan by activated VFF and promoted wound-healing/anti-inflammatory marker expression in activated MΦ. Cumulatively, these data indicate that bFGF-containing hydrogels warrant further investigation for the treatment of vocal fold lamina propria scar. © 2017 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 1258-1267, 2018.
Keywords: 3D cell culture; basic fibroblast growth factor; immunomodulation; vocal fold lamina propria; vocal fold scar.
© 2017 Wiley Periodicals, Inc.
Figures

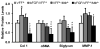
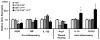
References
-
- Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngologic clinics of North America. 2006;39(1):23–42. - PubMed
-
- Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. Journal of Voice. 2006;20(1):110–120. - PubMed
-
- Graupp M, Bachna-Rotter S, Gerstenberger C, Friedrich G, Fröhlich-Sorger E, Kiesler K, Gugatschka M. The unsolved chapter of vocal fold scars and how tissue engineering could help us solve the problem. European Archives of Oto-Rhino-Laryngology. 2016;273(9):2279–2284. - PubMed
-
- Rosen CA. Phonosurgical vocal fold injection: procedures and materials. Otolaryngologic Clinics of North America. 2000;33(5):1087–1096. - PubMed
-
- Fishman JM, Long J, Gugatschka M, De Coppi P, Hirano S, Hertegard S, Thibeault SL, Birchall MA. Stem cell approaches for vocal fold regeneration. Laryngoscope. 2016;126(8):1865–1870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

