Application of microfluidic devices in studies of thrombosis and hemostasis
- PMID: 28580870
- PMCID: PMC5819608
- DOI: 10.1080/09537104.2017.1319047
Application of microfluidic devices in studies of thrombosis and hemostasis
Abstract
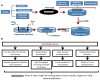
Due to the importance of fluid flow during thrombotic episodes, it is quite appropriate to study clotting and bleeding processes in devices that have well-defined fluid shear environments. Two common devices for applying these defined shear stresses include the cone-and-plate viscometer and parallel-plate flow chamber. While such tools have many salient features, they require large amounts of blood or other protein components. With growth in the area of microfluidics over the last two decades, it has become feasible to miniaturize such flow devices. Such miniaturization not only enables saving of precious samples but also increases the throughput of fluid shear devices, thus enabling the design of combinatorial experiments and making the technique more accessible to the larger scientific community. In addition to simple flows that are common in traditional flow apparatus, more complex geometries that mimic stenosed arteries and the human microvasculature can also be generated. The composition of the microfluidics cell substrate can also be varied for diverse basic science investigations, and clinical investigations that aim to assay either individual patient coagulopathy or response to anti-coagulation treatment. This review summarizes the current state of the art for such microfluidic devices and their applications in the field of thrombosis and hemostasis.
Keywords: PDMS; VWF; complex flow; flow chamber; fluid shear; microfluidics; platelets; point-of-care; stenosis; thrombosis.
Conflict of interest statement
This work was supported by the National Institutes of Health grant HL77258, and American Heart Association award 161RG27770071.
Figures


References
-
- Enderle J, Blanchard S, Bronzino J. Introduction to Biomedical Engineering. San Diego, CA: 2000. Cardiovascular mechanics; pp. 467–535.
-
- Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423–36. - PubMed
-
- Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. 2008;143(2):180–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
