Pyrin-only protein 2 limits inflammation but improves protection against bacteria
- PMID: 28580947
- PMCID: PMC5512670
- DOI: 10.1038/ncomms15564
Pyrin-only protein 2 limits inflammation but improves protection against bacteria
Abstract
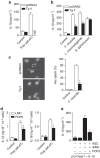
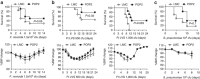
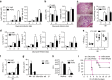
Pyrin domain-only proteins (POPs) are recently evolved, primate-specific proteins demonstrated in vitro as negative regulators of inflammatory responses. However, their in vivo function is not understood. Of the four known POPs, only POP2 is reported to regulate NF-κB-dependent transcription and multiple inflammasomes. Here we use a transgenic mouse-expressing POP2 controlled by its endogenous human promotor to study the immunological functions of POP2. Despite having significantly reduced inflammatory cytokine responses to LPS and bacterial infection, POP2 transgenic mice are more resistant to bacterial infection than wild-type mice. In a pulmonary tularaemia model, POP2 enhances IFN-γ production, modulates neutrophil numbers, improves macrophage functions, increases bacterial control and diminishes lung pathology. Thus, unlike other POPs thought to diminish innate protection, POP2 reduces detrimental inflammation while preserving and enhancing protective immunity. Our findings suggest that POP2 acts as a high-order regulator balancing cellular function and inflammation with broad implications for inflammation-associated diseases and therapeutic intervention.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Takeda K., Kaisho T. & Akira S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003). - PubMed
-
- Martinon F., Burns K. & Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002). - PubMed
-
- Martinon F., Mayor A. & Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

