The particle in the spider's web: transport through biological hydrogels
- PMID: 28580973
- PMCID: PMC5841163
- DOI: 10.1039/c6nr09736g
The particle in the spider's web: transport through biological hydrogels
Abstract
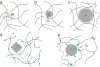
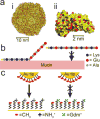
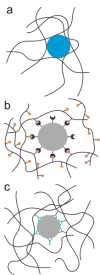
Biological hydrogels such as mucus, extracellular matrix, biofilms, and the nuclear pore have diverse functions and compositions, but all act as selectively permeable barriers to the diffusion of particles. Each barrier has a crosslinked polymeric mesh that blocks penetration of large particles such as pathogens, nanotherapeutics, or macromolecules. These polymeric meshes also employ interactive filtering, in which affinity between solutes and the gel matrix controls permeability. Interactive filtering affects the transport of particles of all sizes including peptides, antibiotics, and nanoparticles and in many cases this filtering can be described in terms of the effects of charge and hydrophobicity. The concepts described in this review can guide strategies to exploit or overcome gel barriers, particularly for applications in diagnostics, pharmacology, biomaterials, and drug delivery.
Figures



References
-
- Orell A, Fröls S, Albers SV. Annu Rev Microbiol. 2013;67:337–354. - PubMed
-
- Flemming HC, Wingender J. Nat Rev Microbiol. 2010;8:623–633. - PubMed
-
- Strambio-De-Castillia C, Niepel M, Rout P. Nat Rev Mol Cell Biol. 2010;11:490–501. - PubMed
-
- Schmidt HB, Görlich D. Trends Biochem Sci. 2016;41:46–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

