Blomia tropicalis allergen 5 (Blo t 5) T-cell epitopes and their ability to suppress the allergic immune response
- PMID: 28581024
- PMCID: PMC5588770
- DOI: 10.1111/imm.12772
Blomia tropicalis allergen 5 (Blo t 5) T-cell epitopes and their ability to suppress the allergic immune response
Erratum in
-
Corrigendum.Immunology. 2018 Mar;153(3):397. doi: 10.1111/imm.12875. Immunology. 2018. PMID: 29392768 Free PMC article. No abstract available.
Abstract
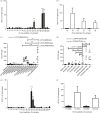
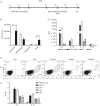
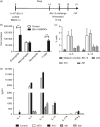
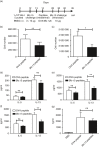
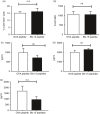
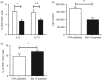
Blomia tropicalis is the major asthma allergen in the tropics comparable to Dermatophagoides pteronyssinus. However, little is known about the B. tropicalis epitopes recognized by T cells. Our aim was to identify the T-cell epitopes in the major B. tropicalis allergen, Blo t 5, and investigate the potential of the corresponding peptides to inhibit the allergic inflammatory lung response. C57BL/6 mice were immunized with plasmid DNA encoding Blo t 5 and T-cell epitopes identified using the interferon-γ ELISPOT assay with 15-mer overlapping peptides. C57BL/6 mice were sensitized with bone-marrow-derived dendritic cells (BMDC) pulsed with Blo t 5 allergen followed by intranasal Blo t 5 challenge. Two H-2b restricted epitopes (Bt576-90 and Bt5106-115 ) were recognized by CD4 T cells specific for Blo t 5, but no CD8 epitopes were identified. In mice sensitized with Blo t 5-pulsed BMDC and challenged with intranasal Blo t 5 Bt576-90 and Bt5106-115 , peptide-specific CD4 T cells were found to secrete the T helper type 2 cytokines interleukin-5 and interleukin-13. Intradermal administration of synthetic peptides encoding the identified T-cell epitopes suppressed allergic airway inflammation to further allergen challenges. Hence, we have identified novel CD4 T-cell epitopes specific for Blo t 5 and demonstrated that these peptides could be employed therapeutically to suppress the T-cell response in a murine model of allergic airway inflammation.
Keywords: Allergy; Blomia tropicalis; DNA vaccine; mouse model.
© 2017 John Wiley & Sons Ltd.
Figures
References
-
- Yeoh SM, Kuo IC, Wang DY, Liam CK, Sam CK, de Bruyne JA et al Sensitization profiles of Malaysian and Singaporean subjects to allergens from Dermatophagoides pteronyssinus and Blomia tropicalis . Int Arch Allergy Immunol 2003; 132:215–20. - PubMed
-
- Arruda LK, Vailes LD, Platts‐Mills TA, Fernandez‐Caldas E, Montealegre F, Lin KL et al Sensitization to Blomia tropicalis in patients with asthma and identification of allergen Blo t 5. Am J Respir Crit Care Med 1997; 155:343–50. - PubMed
-
- Yi FC, Cheong N, Shek LP, Wang DY, Chua KY, Lee BW. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus . Clin Exp Allergy 2002; 32:1203–10. - PubMed
-
- Simpson A, Green R, Custovic A, Woodcock A, Arruda LK, Chapman MD. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy 2003; 58:53–6. - PubMed
-
- Kuo IC, Cheong N, Trakultivakorn M, Lee BW, Chua KY. An extensive study of human IgE cross‐reactivity of Blo t 5 and Der p 5. J Allergy Clin Immunol 2003; 111:603–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

