Prevalence of hepatitis B antiviral drug resistance variants in North American patients with chronic hepatitis B not receiving antiviral treatment
- PMID: 28581155
- PMCID: PMC5638682
- DOI: 10.1111/jvh.12732
Prevalence of hepatitis B antiviral drug resistance variants in North American patients with chronic hepatitis B not receiving antiviral treatment
Abstract
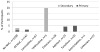
Antiviral drug resistance hepatitis B virus (HBV) variants (HBV-DR) occur spontaneously in chronic hepatitis B (CHB) patients and after exposure to nucleos(t)ide analogues (NUCs). We determined the prevalence of HBV-DR variants among participants of the Hepatitis B Research Network (HBRN) Cohort Study conducted at 21 sites in the United States (US) and Canada. Samples obtained from 1342 CHB participants aged ≥18 years, and who were currently not receiving NUCs, were tested for HBV-DR variants by Sanger sequencing. In addition, next generation sequencing (NGS) was used to characterize HBV-DR variants from 66 participants with and 66 participants with no prior NUC exposure matched for HBV genotype and HBV DNA level. Half the participants were men, 75% Asian, 26% HBeAg positive. Primary HBV-DR variants were detected by Sanger sequencing in 16 (1.2%) participants: 2/142 (1.4%) with and 14/1200 (1.2%) without prior NUC exposure; only 1 of these 16 had a secondary variant. In total, 23 (1.7%) participants had secondary variants, including 1 with prior NUC experience. In the subset of 132 participants, NGS detected HBV-DR variants in a higher proportion of participants: primary variants in 18 (13.6%) (8 [12.1%] with, and 10 [15.2%] without prior NUC therapy) and secondary variants in 10 (7.6%) participants. Based on Sanger sequencing, prevalence of primary HBV-DR variants is low (1.2%) among adults with CHB in US/Canada. The similar low prevalence of HBV-DR variants in participants with and without NUC treatment suggests transmission of these variants is uncommon.
Keywords: Sanger sequencing; antiviral treatment; hepatitis B virus; next generation sequencing; nucleos(t)ide analogues.
© 2017 John Wiley & Sons Ltd.
Figures
References
-
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. - PubMed
-
- Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137(5):1593–608. e1–2. - PubMed
-
- Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46(1):254–65. - PubMed
-
- Teo CG, Locarnini SA. Potential threat of drug-resistant and vaccine-escape HBV mutants to public health. Antivir Ther. 2010;15(3 Pt B):445–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- UL1 TR001111/TR/NCATS NIH HHS/United States
- U01 DK082923/DK/NIDDK NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- U01 DK082867/DK/NIDDK NIH HHS/United States
- U01 DK082874/DK/NIDDK NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK082919/DK/NIDDK NIH HHS/United States
- U01 DK082927/DK/NIDDK NIH HHS/United States
- U01 DK082872/DK/NIDDK NIH HHS/United States
- U01 DK082943/DK/NIDDK NIH HHS/United States
- K24 AA022523/AA/NIAAA NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- U01 DK082871/DK/NIDDK NIH HHS/United States
- U01 DK082944/DK/NIDDK NIH HHS/United States
- U01 DK082864/DK/NIDDK NIH HHS/United States
- U01 DK082843/DK/NIDDK NIH HHS/United States
- U01 DK082863/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK082866/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

