Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis
- PMID: 28581444
- PMCID: PMC5490758
- DOI: 10.1172/JCI90592
Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis
Abstract
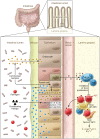
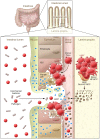
Lower gastrointestinal (GI) tract graft-versus-host disease (GVHD) is the predominant cause of morbidity and mortality from GVHD after allogeneic stem cell transplantation. Recent data indicate that lower GI tract GVHD is a complicated process mediated by donor/host antigenic disparities. This process is exacerbated by significant changes to the microbiome, and innate and adaptive immune responses that are critical to the induction of disease, persistence of inflammation, and a lack of response to therapy. Here, we discuss new insights into the biology of lower GI tract GVHD and focus on intrinsic pathways and regulatory mechanisms crucial to normal intestinal function. We then describe multiple instances in which these homeostatic mechanisms are altered by donor T cells or conditioning therapy, resulting in exacerbation of GVHD. We also discuss data suggesting that some of these mechanisms produce biomarkers that could be informative as to the severity of GVHD and its response to therapy. Finally, novel therapies that might restore homeostasis in the GI tract during GVHD are highlighted.
Conflict of interest statement
Figures
Similar articles
-
Acute Graft-versus-Host Disease: Novel Biological Insights.Biol Blood Marrow Transplant. 2016 Jan;22(1):11-6. doi: 10.1016/j.bbmt.2015.10.001. Epub 2015 Oct 26. Biol Blood Marrow Transplant. 2016. PMID: 26453971 Review.
-
Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease.J Clin Invest. 2017 May 1;127(5):1813-1825. doi: 10.1172/JCI91816. Epub 2017 Apr 4. J Clin Invest. 2017. PMID: 28375154 Free PMC article.
-
The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease.J Endotoxin Res. 2002;8(6):441-8. doi: 10.1179/096805102125001046. J Endotoxin Res. 2002. PMID: 12697087 Review.
-
Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy.Blood. 1990 Aug 1;76(3):624-9. Blood. 1990. PMID: 2378989
-
Monogenic Immune Diseases Provide Insights Into the Mechanisms and Treatment of Chronic Graft-Versus-Host Disease.Front Immunol. 2021 Feb 4;11:574569. doi: 10.3389/fimmu.2020.574569. eCollection 2020. Front Immunol. 2021. PMID: 33613511 Free PMC article. Review.
Cited by
-
Placenta-Derived Decidua Stromal Cells: A New Frontier in the Therapy of Acute Graft-Versus-Host Disease.Stem Cells. 2024 Apr 15;42(4):291-300. doi: 10.1093/stmcls/sxae003. Stem Cells. 2024. PMID: 38204331 Free PMC article.
-
The Absence of IL-12Rβ2 Expression on Recipient Nonhematopoietic Cells Diminishes Acute Graft-versus-Host Disease in the Gastrointestinal Tract.J Immunol. 2023 Feb 15;210(4):486-495. doi: 10.4049/jimmunol.2200120. J Immunol. 2023. PMID: 36548465 Free PMC article.
-
High-density lipoprotein infusion protects from acute graft-versus-host disease in experimental allogeneic hematopoietic cell transplantation.Am J Transplant. 2022 May;22(5):1350-1361. doi: 10.1111/ajt.16960. Epub 2022 Feb 10. Am J Transplant. 2022. PMID: 35038785 Free PMC article.
-
Inflammation-induced epigenetic imprinting regulates intestinal stem cells.Cell Stem Cell. 2024 Oct 3;31(10):1447-1464.e6. doi: 10.1016/j.stem.2024.08.006. Epub 2024 Sep 3. Cell Stem Cell. 2024. PMID: 39232559
-
[Effects of donor T cell stat3 deficiency on acute intestinal graft-versus-host disease in mice].Zhonghua Xue Ye Xue Za Zhi. 2025 Apr 14;46(4):302-313. doi: 10.3760/cma.j.cn121090-20250107-00011. Zhonghua Xue Ye Xue Za Zhi. 2025. PMID: 40425451 Free PMC article. Chinese.
References
-
- Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. - PubMed
-
- Gatti RA, Kersey JH, Yunis EJ, Good RA. Graft-versus-host disease. Prog Clin Pathol. 1973;5:1–18. - PubMed
-
- Benacerraf B, Kapp JA, Pierce CW, Katz DH. Genetic control of immune responses in vitro. IV. Conditions for cooperative interactions between nonresponder parental B cells and primed (responder plus nonresponder) F1 T cells in the development of an antibody response under Ir gene control in vitro. J Exp Med. 1974;140(1):185–198. doi: 10.1084/jem.140.1.185. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

