Tregs restrain dendritic cell autophagy to ameliorate autoimmunity
- PMID: 28581446
- PMCID: PMC5490766
- DOI: 10.1172/JCI92079
Tregs restrain dendritic cell autophagy to ameliorate autoimmunity
Abstract
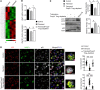
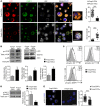
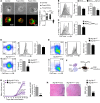
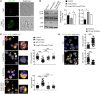
Design of efficacious Treg-based therapies and establishment of clinical tolerance in autoimmune diseases have proven to be challenging. The clinical implementation of Treg immunotherapy has been hampered by various impediments related to the stability and isolation procedures of Tregs as well as the specific in vivo targets of Treg modalities. Herein, we have demonstrated that Foxp3+ Tregs potently suppress autoimmune responses in vivo through inhibition of the autophagic machinery in DCs in a cytotoxic T-lymphocyte-associated protein 4-dependent (CTLA4-dependent) manner. Autophagy-deficient DCs exhibited reduced immunogenic potential and failed to prime autoantigen-specific CD4+ T cells to mediate autoimmunity. Mechanistically, CTLA4 binding promoted activation of the PI3K/Akt/mTOR axis and FoxO1 nuclear exclusion in DCs, leading to decreased transcription of the autophagy component microtubule-associated protein 1 light chain 3β (Lc3b). Human DCs treated with CTLA4-Ig, a fusion protein composed of the Fc region of IgG1 and the extracellular domain of CTLA4 (also known as abatacept, marketed as Orencia), demonstrated reduced levels of autophagosome formation, while DCs from CTLA4-Ig-treated rheumatoid arthritis patients displayed diminished LC3B transcripts. Collectively, our data identify the canonical autophagy pathway in DCs as a molecular target of Foxp3+ Treg-mediated suppression that leads to amelioration of autoimmune responses. These findings may pave the way for the development of therapeutic protocols that exploit Tregs for the treatment of autoimmunity as well as diseases in which disturbed tolerance is a common denominator.
Conflict of interest statement
Figures

Comment in
-
Immunology: Inhibiting autophagy in dendritic cells.Nat Rev Rheumatol. 2017 Aug;13(8):452. doi: 10.1038/nrrheum.2017.101. Epub 2017 Jun 22. Nat Rev Rheumatol. 2017. PMID: 28660914 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

