Designing microorganisms for heterologous biosynthesis of cannabinoids
- PMID: 28582498
- PMCID: PMC5812543
- DOI: 10.1093/femsyr/fox037
Designing microorganisms for heterologous biosynthesis of cannabinoids
Abstract
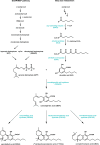
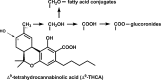
During the last decade, the use of medical Cannabis has expanded globally and legislation is getting more liberal in many countries, facilitating the research on cannabinoids. The unique interaction of cannabinoids with the human endocannabinoid system makes these compounds an interesting target to be studied as therapeutic agents for the treatment of several medical conditions. However, currently there are important limitations in the study, production and use of cannabinoids as pharmaceutical drugs. Besides the main constituent tetrahydrocannabinolic acid, the structurally related compound cannabidiol is of high interest as drug candidate. From the more than 100 known cannabinoids reported, most can only be extracted in very low amounts and their pharmacological profile has not been determined. Today, cannabinoids are isolated from the strictly regulated Cannabis plant, and the supply of compounds with sufficient quality is a major problem. Biotechnological production could be an attractive alternative mode of production. Herein, we explore the potential use of synthetic biology as an alternative strategy for synthesis of cannabinoids in heterologous hosts. We summarize the current knowledge surrounding cannabinoids biosynthesis and present a comprehensive description of the key steps of the genuine and artificial pathway, systems biotechnology needs and platform optimization.
Keywords: Cannabis sativa; Saccharomyces cerevisiae; biotechnology; cannabinoids; synthetic biology.
© FEMS 2017.
Figures

References
-
- Akhtar MT, Mustafa NR, Verpoorte R. Hydroxylation and glycosylation of Δ9-tetrahydrocannabinol by Catharanthus roseus cell suspension culture. Biocatal Biotransfor 2015;33:279–86.
-
- Appendino G, Gibbons S, Giana A et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod 2008;71:1427–30. - PubMed
-
- Aritomi K, Hirosawa I, Hoshida H et al. Self-cloning yeast strains containing novel FAS2 mutations produce a higher amount of ethyl caproate in Japanese sake. Biosci Biotech Bioch 2004;68:206–14. - PubMed
-
- Binder M, Meisenberg G. Microbial transformation of cannabinoids - part 2; a screening of different microorganisms. Eur J Appl Microbiol 1978;5:37–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

