The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae
- PMID: 28582571
- PMCID: PMC5853415
- DOI: 10.1093/jxb/erx179
The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae
Abstract
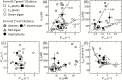
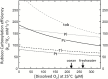
The haptophyte algae are a cosmopolitan group of primary producers that contribute significantly to the marine carbon cycle and play a major role in paleo-climate studies. Despite their global importance, little is known about carbon assimilation in haptophytes, in particular the kinetics of their Form 1D CO2-fixing enzyme, Rubisco. Here we examine Rubisco properties of three haptophytes with a range of pyrenoid morphologies (Pleurochrysis carterae, Tisochrysis lutea, and Pavlova lutheri) and the diatom Phaeodactylum tricornutum that exhibit contrasting sensitivities to the trade-offs between substrate affinity (Km) and turnover rate (kcat) for both CO2 and O2. The pyrenoid-containing T. lutea and P. carterae showed lower Rubisco content and carboxylation properties (KC and kCcat) comparable with those of Form 1D-containing non-green algae. In contrast, the pyrenoid-lacking P. lutheri produced Rubisco in 3-fold higher amounts, and displayed a Form 1B Rubisco kCcat-KC relationship and increased CO2/O2 specificity that, when modeled in the context of a C3 leaf, supported equivalent rates of photosynthesis to higher plant Rubisco. Correlation between the differing Rubisco properties and the occurrence and localization of pyrenoids with differing intracellular CO2:O2 microenvironments has probably influenced the divergent evolution of Form 1B and 1D Rubisco kinetics.
Keywords: Algae; Haptophyta; Rubisco; carbon-concentrating mechanisms; pyrenoid.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based. Canadian Journal of Botany 1071, 1052–1071.
-
- Badger MR, Hanson D, Price GD. 2002. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Functional Plant Biology 29, 161–173. - PubMed
-
- Bedoshvili YD, Popkova TP, Likhoshway YV. 2009. Chloroplast structure of diatoms of different classes. Cell and Tissue Biology 3, 297–310.
-
- Beech PL, Wetherbee R. 1988. Observations on the flagellar apparatus and peripheral endoplasmic reticulum of the coccolithophorid, Pleurochrysis carterae (Prymnesiophyceae). Phycologia 27, 142–158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

