Diagnostic accuracy of nucleic acid amplification tests (NAATs) in urine for genitourinary tuberculosis: a systematic review and meta-analysis
- PMID: 28583076
- PMCID: PMC5460328
- DOI: 10.1186/s12879-017-2476-8
Diagnostic accuracy of nucleic acid amplification tests (NAATs) in urine for genitourinary tuberculosis: a systematic review and meta-analysis
Abstract
Background: Genitourinary tuberculosis is the third most common form of extrapulmonary tuberculosis. Diagnosis is difficult because of unspecific clinical manifestations and low accuracy of conventional tests. Unfortunately, the delayed diagnosis impacts the urinary tract severely. Nucleic acid amplification tests yield fast results, and among these, new technologies can also detect drug resistance. There is lack of consensus regarding the use of these tests in genitourinary tuberculosis; we therefore aimed to assess the accuracy of nucleic acid amplification tests in the diagnosis of genitourinary tuberculosis and to evaluate the heterogeneity between studies.
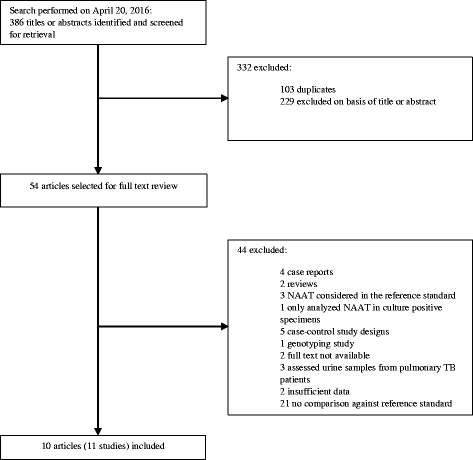
Methods: We did a systematic review and meta-analysis of research articles comparing the accuracy of a reference standard and a nucleic acid amplification test for diagnosis of urinary tract tuberculosis. We searched Medline, EMBASE, Web of Science, LILACS, Cochrane Library, and Scopus for articles published between Jan 1, 1990, and Apr 14, 2016. Two investigators identified eligible articles and extracted data for individual study sites. We analyzed data in groups with the same index test. Then, we generated pooled summary estimates (95% CIs) for sensitivity and specificity by use of random-effects meta-analysis when studies were not heterogeneous.
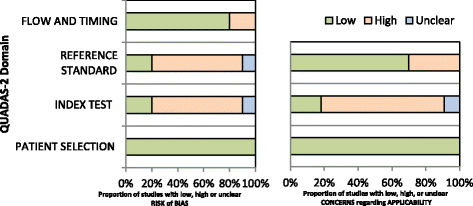
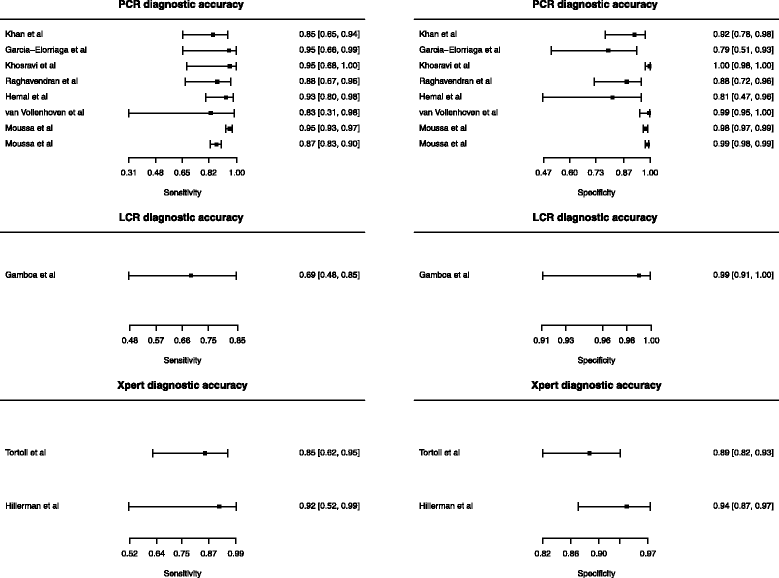
Results: We identified eleven relevant studies from ten articles, giving information on PCR, LCR and Xpert MTB/RIF tests. All PCR studies were "in-house" tests, with different gene targets and had several quality concerns therefore we did not proceed with a pooled analysis. Only one study used LCR. Xpert studies were of good quality and not heterogeneous, pooled sensitivity was 0·87 (0·66-0·96) and specificity was 0·91 (0·84-0·95).
Conclusion: PCR studies were highly heterogeneous. Among Xpert MTB/RIF studies, specificity was favorable with an acceptable confidence interval, however new studies can update meta-analysis and get more precise estimates. Further high-quality studies are urgently needed to improve diagnosis of genitourinary tuberculosis.
Protocol registration: PROSPERO CRD42016039020.
Keywords: Genitourinary tuberculosis; Nucleic acid amplification test; Systematic review.
Figures

Similar articles
-
Clinical validation of urine-based Xpert® MTB/RIF assay for the diagnosis of urogenital tuberculosis: A systematic review and meta-analysis.Int J Infect Dis. 2020 Jun;95:15-21. doi: 10.1016/j.ijid.2020.03.023. Epub 2020 Mar 17. Int J Infect Dis. 2020. PMID: 32194240
-
Commercial nucleic acid amplification tests in tuberculous meningitis--a meta-analysis.Diagn Microbiol Infect Dis. 2014 Apr;78(4):398-403. doi: 10.1016/j.diagmicrobio.2014.01.002. Epub 2014 Jan 10. Diagn Microbiol Infect Dis. 2014. PMID: 24503504
-
Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis.Cochrane Database Syst Rev. 2021 May 6;5(5):CD012972. doi: 10.1002/14651858.CD012972.pub2. Cochrane Database Syst Rev. 2021. PMID: 34097769 Free PMC article.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2019 Jun 7;6(6):CD009593. doi: 10.1002/14651858.CD009593.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 22;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. PMID: 31173647 Free PMC article. Updated.
-
Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms.Cochrane Database Syst Rev. 2021 Mar 23;3(3):CD013694. doi: 10.1002/14651858.CD013694.pub2. Cochrane Database Syst Rev. 2021. PMID: 33755189 Free PMC article.
Cited by
-
The Xpert® MTB/RIF diagnostic test for pulmonary and extrapulmonary tuberculosis in immunocompetent and immunocompromised patients: Benefits and experiences over 2 years in different clinical contexts.PLoS One. 2021 Mar 3;16(3):e0247185. doi: 10.1371/journal.pone.0247185. eCollection 2021. PLoS One. 2021. PMID: 33657113 Free PMC article. Clinical Trial.
-
Multicentre evaluation of Xpert MTB/RIF assay in detecting urinary tract tuberculosis with urine samples.Sci Rep. 2019 Jul 30;9(1):11053. doi: 10.1038/s41598-019-47358-3. Sci Rep. 2019. PMID: 31363115 Free PMC article.
-
A Case Report of Renal Tuberculosis With Associated Unusual Pulmonary Findings.Cureus. 2021 Nov 28;13(11):e19972. doi: 10.7759/cureus.19972. eCollection 2021 Nov. Cureus. 2021. PMID: 34984132 Free PMC article.
-
Performance of Various Pelvic-Derived Samples for the Diagnosis of Female Genital Tract Tuberculosis by Conventional and Molecular Methods: A Systematic Review and Meta-Analysis.Cureus. 2025 May 13;17(5):e84016. doi: 10.7759/cureus.84016. eCollection 2025 May. Cureus. 2025. PMID: 40519447 Free PMC article. Review.
-
Decoding the hidden realm: Molecular pioneering unravelling osteoarticular tuberculosis diagnosis.J Clin Orthop Trauma. 2024 Sep 12;56:102538. doi: 10.1016/j.jcot.2024.102538. eCollection 2024 Sep. J Clin Orthop Trauma. 2024. PMID: 39318541
References
-
- World Health Organization (WHO). Global Tuberculosis Report. Ginebra: WHO. 2015:2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

