Effect sizes in ongoing randomized controlled critical care trials
- PMID: 28583149
- PMCID: PMC5460326
- DOI: 10.1186/s13054-017-1726-x
Effect sizes in ongoing randomized controlled critical care trials
Abstract
Background: An important limitation of many critical care trial designs is that they hypothesize large, and potentially implausible, reductions in mortality. Interpretation of trial results could be improved by systematic assessment of the plausibility of trial hypotheses; however, such assessment has not been attempted in the field of critical care medicine. The purpose of this study was to determine clinicians' views about prior probabilities and plausible effect sizes for ongoing critical care trials where the primary endpoint is landmark mortality.
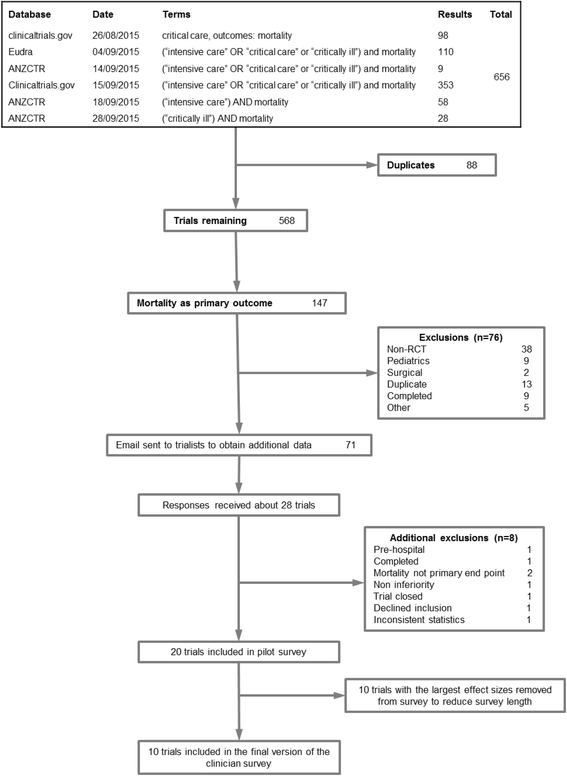
Methods: We conducted a systematic review of clinical trial registries in September 2015 to identify ongoing critical care medicine trials where landmark mortality was the primary outcome, followed by a clinician survey to obtain opinions about ten large trials. Clinicians were asked to estimate the probability that each trial would demonstrate a mortality effect equal to or larger than that used in its sample size calculations.
Results: Estimates provided by individual clinicians varied from 0% to 100% for most trials, with a median estimate of 15% (IQR 10-20%). The median largest absolute mortality reduction considered plausible was 4.5% (IQR 3.5-5%), compared with a median absolute mortality reduction used in sample size calculations of 5% (IQR 3.6-10%) (P = 0.27).
Conclusions: For some of the largest ongoing critical care trials, many clinicians regard prior probabilities as low and consider that plausible effects on absolute mortality are less than 5%. Further work is needed to determine whether pooled estimates obtained by surveying clinicians are replicable and accurate or whether other methods of estimating prior probability are preferred.
Keywords: Clinical trial design; Critical care; Intensive care; Intensive care unit; Randomized clinical trial.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

